Surveillance for Transmissible Spongiform Encephalopathy in Scavengers of White-Tailed Deer Carcasses in the Chronic Wasting Disease Area
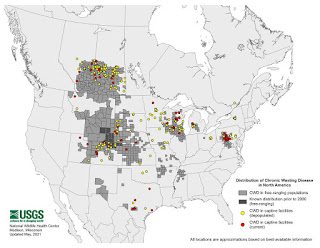
CWD MAP
Title: Surveillance for Transmissible Spongiform Encephalopathy in Scavengers of White-Tailed Deer Carcasses in the Chronic Wasting Disease Area of Wisconsin
Author item JENNELLE, CHRISTOPHER - University Of Wisconsin item SAMUEL, MICHAEL - Us Geological Survey (USGS) item NOLDEN, CHERRIE - University Of Wisconsin item KEANE, DELWYN - University Of Wisconsin item BARR, DANIEL - University Of Wisconsin item JOHNSON, CHAD - University Of Wisconsin item VANDERLOO, JOSHUA - University Of Wisconsin item AIKEN, JUDD - University Of Wisconsin item Hamir, Amirali item HOOVER, EDWARD - Colorado State University Submitted to: Journal of Toxicology and Environmental Health Publication Type: Peer Reviewed Journal Publication Acceptance Date: 9/1/2009 Publication Date: 1/1/2009 Citation: Jennelle, C.S., Samuel, M.D., Nolden, C.A., Keane, D.P., Barr, D.J., Johnson, C., Vanderloo, J.P., Aiken, J.M., Hamir, A.N., Hoover, E.A. 2009. Surveillance for Transmissible Spongiform Encephalopathy in Scavengers of White-Tailed Deer Carcasses in the Chronic Wasting Disease Area of Wisconsin. Journal of Toxicology and Environmental Health, Part A. 72(17):1018-1024.
Interpretive Summary: Chronic wasting disease (CWD), a neurologic disease, occurring in cervids, is found in a number of states and provinces across North America. Infected material containing the infectious agents of CWD, are deposited in the environment via carcass remains and excreta, and pose a threat of cross-species transmission. In this study tissues were tested from 812 representative mammalian scavengers, collected in the CWD-infected area of Wisconsin, for TSE infection using commercial and laboratory tests. The commercial test found four samples to be positive; but these samples were negative by the superior laboratory test. Based on these findings, one cannot rule out successful cross-species TSE transmission to scavengers, but the results suggest that such transmission is not frequent in the CWD-affected area of Wisconsin.
Technical Abstract: Chronic wasting disease (CWD), a class of neurodegenerative transmissible spongiform encephalopathies (TSE) occurring in cervids, is found in a number of states and provinces across North America. Misfolded prions, the infectious agents of CWD, are deposited in the environment via carcass remains and excreta, and pose a threat of cross-species transmission. In this study tissues were tested from 812 representative mammalian scavengers, collected in the CWD-infected area of Wisconsin, for TSE infection using the IDEXX HersCheck enzyme-linked immuosorbent assay (ELISA). Only four of the collected mammals tested positive using ELISA, but these were negative when tested by Western blot. While our sample sizes permitted high probabilities of detecting TSE assuming 1% population prevalence in several common scavengers (93%, 87%, and 87% for raccoons, opossums, and coyotes, respectively), insufficient sample sizes for other species precluded similar conclusions. One cannot rule out successful cross-species TSE transmission to scavengers, but the results suggest that such transmission is not frequent in the CWD-affected area of Wisconsin. The need for further surveillance of scavenger species, especially those known to be susceptible to TSE (e.g. cat, American mink, raccoon), is highlighted in both a field and laboratory setting.
Wisconsin Buckhorn Flats CWD
SUBJECT: Almond Deer Farm Update
The first case of Chronic Wasting Disease (CWD) among Wisconsin's farm-raised deer occurred in a white-tailed deer buck shot by a hunter at the property (formerly known as Buckhorn Flats) in September 2002. This situation prompted the eventual depopulation of the entire farm.
The deer, a mix of does and yearlings, were destroyed on January 17, 2006- 4 years later- by U.S. Department of Agriculture shooters under a USDA agreement with the farm owner.
Sixty of the 76 animals tested positive for CWD. The 76 deer constituted the breeding herd in the breeding facility on the farm. The property also had a hunting preserve until 2005. Four deer, two does and two fawns, the only deer remaining in the former preserve, were killed and tested as well. CWD was not detected in those animals.
The total number of deer to test positive from this farm from the initial discovery to final depopulation is 82. The nearly 80% prevalence rate discovered on Buckhorn Flats is the highest prevalence recorded in any captive cervid operation in North America.
Tuesday, December 20, 2011
Chronic Wasting Disease CWD WISCONSIN Almond Deer
(Buckhorn Flats) Farm Update DECEMBER 2011 The CWD infection rate was nearly 80%, the highest ever in a North American captive herd. RECOMMENDATION: That the Board approves the purchase of 80 acres of land for $465,000 for the Statewide Wildlife Habitat Program in Portage County and approve the restrictions on public use of the site.
Form 1100-001 (R 2/11) NATURAL RESOURCES BOARD AGENDA ITEM SUBJECT: Information Item: Almond Deer Farm Update FOR:
DECEMBER 2011 BOARD MEETING
TUESDAY TO BE PRESENTED BY TITLE: Tami Ryan, Wildlife Health Section Chief SUMMARY:
THURSDAY, MARCH 18, 2021
Wisconsin Burnett County Deer Herd Depopulated Due to CWD
TUESDAY, JUNE 09, 2020
Wisconsin Trempealeau County Deer Farm Tests Positive for CWD Release Date: June 9, 2020
TUESDAY, JANUARY 12, 2021
Wisconsin CWD TSE Prion 8,101 Positive With Wild Deer Testing Positive for CWD in Germania in Southwestern Shawano County
***> CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans) <***
Tracy A Nichols,Justin W Fischer,Terry R Spraker,Qingzhong Kong &Kurt C VerCauteren
Pages 367-375 | Received 07 Jul 2015, Accepted 18 Aug 2015, Accepted author version posted online: 04 Dec 2015, Published online:21 Dec 2015
Download citation https://doi.org/10.1080/19336896.2015.1086061
ABSTRACT
Chronic wasting disease (CWD) is a geographically expanding prion disease of wild and captive cervids in North America. Disease can be transmitted directly, animal to animal, or indirectly via the environment. CWD contamination can occur residually in the environment via soil, water, and forage following deposition of bodily fluids such as urine, saliva, and feces, or by the decomposition of carcasses. Recent work has indicated that plants may even take up prions into the stems and leaves. When a carcass or gut pile is present in the environment, a large number of avian and mammalian species visit and consume the carrion. Additionally, predators like coyotes, likely select for disease-compromised cervids. Natural cross-species CWD transmission has not been documented, however, passage of infectious prion material has been observed in the feces of crows. In this study we evaluated the ability of CWD-infected brain material to pass through the gastrointestinal tract of coyotes (Canis latrans) following oral ingestion, and be infectious in a cervidized transgenic mouse model. Results from this study indicate that coyotes can pass infectious prions via their feces for at least 3 days post ingestion, demonstrating that mammalian scavengers could contribute to the translocation and contamination of CWD in the environment.
Introduction Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy, or prion disease, of deer (Odocoileus virginianus and O. hemionus), elk (Cervus elaphus) and moose (Alces alces). First identified in 1967 in a wildlife research center in Colorado, CWD is the only prion disease enzootic to wild animals.1 Transmission of CWD can occur directly, animal to animal,2 or indirectly through the environment.3 Contamination of the environment can occur by deposition of bodily fluids4-6 or by decay of infected carcasses.3 Ingestion or inhalation of contaminated soil particles can also lead to disease transmission.7,8
Each year the number of states reporting incidences of CWD in captive or wild cervid populations increases. Currently, 21 states have been affected (http://www.cwd-info.org/index.php/fuseaction/about.map). In some regions the spread has been contiguous, such as that seen in Colorado, Wyoming, and Nebraska. Other incidences are far removed from known CWD-positive foci. The mechanisms for this expansion are unclear, and likely vary by circumstance. Several human behaviors, such as movement of captive cervids9 and the dumping of CWD-positive carcasses from hunter kills in CWD-negative regions,10 have likely contributed to the expansion, but may not explain all incidences.
The role scavengers play in the spread of the disease has been evaluated primarily from a cross-species transmission aspect.11,12 A wide variety of avian and mammalian scavenger species have been documented to feed upon deer carcasses and gut piles.12 The array of tissues that contain CWD include brain, eyes, lymph nodes, neural tissue, heart, spleen, and muscle,13-17 and are all readily accessible in both carcasses and gut piles. Common scavengers from CWD-enzootic areas (raccoons (Procyon lotor), opossums (Didelphis virginiana) and coyotes (Canis latrans)) have been evaluated for the presence of CWD in their tissues, but no evidence of CWD was detected, suggesting that they do not play a direct role in transmission or become infected.11 They may, however, play a more indirect role. Recent work demonstrated that infectious mouse-adapted scrapie prions could be viably passed in the feces of crows (Corvus brachyrrhynchos) after ingestion.10 Deposition of infectious feces from scavengers could then be another unexplored mode for environmental contamination. Mammalian scavengers, such as coyotes, are of particular interest in western states such as Colorado and Wyoming, where there are both a high number of CWD-infected deer and elk, and coyotes.
Coyotes are opportunistic and widespread carnivores found throughout much of North America and everywhere CWD is enzootic in the wild.18 Their diet is composed primarily of rodents and lagomorphs, however, diet composition can vary seasonally and by geographical location and include ungulates.19,20 For example, coyotes in the Black Hills of South Dakota feed preferentially on white-tailed deer (Odocoileus virginianus) throughout the year, with the highest consumption occurring (72%) in the winter.21 In addition to predation, coyotes will opportunistically forage on carcasses and entrails left by human hunters.22-24 Both predation and scavenging exposes coyotes to CWD in affected regions.
Little is known about the degradation of CWD-infected tissue and infectivity after passage through the gastrointestinal tract of mammalian scavengers. In this study we investigated the potential for coyotes to translocate infectious CWD prions, via feces, after oral consumption of CWD-infected elk brain, utilizing a cervidized transgenic mouse bioassay.
snip...
Discussion The continued spread of CWD is of concern to the health of both wild and captive cervid populations. Indirect transmission through the environment has been demonstrated in captive animals living in paddocks where CWD-positive animals had lived,3 and is a particular challenge due to the long persistence of CWD within the environment.7,28 Infectious material can be deposited in the environment by the decay of infected carcasses, from urine, feces, and saliva,5,6,29 and the spread of infected material may be aided by scavengers and predators. In this study we illustrated the ability of coyotes to pass infectivity in their feces after the ingestion of CWD-infected brain homogenate.
Coyotes have the ability to travel significant distances. This distance, however, is based upon social structure, which is generally placed in 2 categories; resident or transient.30 Resident animals are those that utilize a specific territory and are comprised of a mated pair and sometimes pups from a previous year, while transient animals are individuals that are nomadic, more commonly male, and have no affinity for a specific territory.30 In a study evaluating the range of coyotes in southern Colorado, transient animals, which represented 22% of the population, ranged over 106.5 ± 27 km2, versus resident groups which ranged over 11.3 ± 5.8 km.2,30 Transient coyotes are therefore provided an opportunity to translocate disease to previously CWD-negative localities.
Control coyotes readily consumed the homogenized elk brain. Of the treatment coyotes, which were moved indoors 2 days prior to the initiation of the study, only one (#135) immediately ate the brain homogenate. The other coyotes required supplementation with diced, raw chicken, or fish-flavored soft cat food. Although the numbers are too small to come to any definitive conclusions, it is interesting to note that the coyote that ingested the brain homogenate without chicken or cat food supplementation did not appear to transfer infectivity to any of the mice in the bioassay. Neither age nor sex appeared to have any effect on fecal shedding. However, it is possible that individual variation within the stomach environment, such as pH and flora could have influenced the passage of the infectious prions through the gastrointestinal tract.
Our experimental design was based on detection of CWD in coyote feces by PMCA prior to initiation of the bioassay. PMCA was able to repeatedly detect the presence of proteinase K-resistant prions signal in feces from DPI 1, so the bioassay was designed to evaluate feces for 2 days following, to account for any uncertainty in prion detection in feces. Results from the bioassay showed transmission of disease to 2/4 mouse groups in DPI 3, suggesting that infectivity may continue to be present in the feces more than 3 days after ingestion. We were unable to go back and increase the bioassay to include DPI 4 and 5, due to logistical reasons.
The 50 mL oral dose ingested by coyotes in this study was comprised solely of infected brain tissue and represented a high dose. In the wild, coyotes would opportunistically consume a wide variety of tissues from a kill or scavenged deer or elk carcass, likely making their actual ingested infective dose much smaller. This study was not designed to mimic a naturally consumed dose of CWD, but rather as a proof of concept to determine if infectivity could pass into coyote feces. The passage of disease in feces is a common route of translocation for many viral, bacterial and parasitic diseases.
The results of this bioassay indicate that infectious CWD prions are able to be passed in the feces of coyotes fed infected elk brain homogenate for at least 3 DPI, making them a potential vector for CWD prion transport and contamination within the environment.
MONDAY, NOVEMBER 16, 2020
North America coyotes or pumas can serve as a vehicle for prions contributing to the spread of the infectious agent in the environment
News Release
U of M testing finds presence of CWD prions at Beltrami County carcass dump site
May 18, 2021
Using forensic science techniques, a team led by University of Minnesota scientists recovered samples from a remote Beltrami County site used by a nearby deer farm to discard white-tailed deer carcasses. Testing for the presence of chronic wasting disease (CWD)-causing prions has found one bone marrow sample to be positive.
The rapid testing was completed using RT-QuIC technology, a highly-sensitive assay that can be used to identify CWD prions in carcasses and the environment. Faster, accurate testing that can be used on a wide variety of sample types is critical to improving efforts to limit the spread of CWD, a transmissible neurological disease that is always fatal to white-tailed deer.
“This is a rapidly evolving situation. We are glad that we were able to assist our collaborators at the Minnesota Department of Natural Resources, the Minnesota Board of Animal Health, and the U.S. Department of Agriculture with RT-QuIC testing of the carcasses,” said Peter Larsen, Ph.D., who led the team and co-directs the Minnesota Center for Prion Research and Outreach (MNPRO) at the University. “Our work helps everyone respond more quickly with actions to safeguard our collective white-tailed deer resources. Identification of a positive carcass within an area that is frequented by wild white-tailed deer is highly concerning. Our MNPRO team is ready to assist with securing the dump-site to try and prevent CWD from spreading to the surrounding wild herds.”
Sweeping across the site on May 2, the team collected bones, hides, soil and plant samples. Their expertise in cervid anatomy and mortality investigations of wildlife allowed the discovery of portions of ten or more deer. Additionally, the team’s knowledge of the conditions that promote the survival of CWD-causing prions allowed them to focus on collecting and processing samples obtained from highly deteriorated and desiccated materials with a high likelihood of retaining the prions months or years after their deposition.
The nearby deer farm herd was depopulated last week, and samples from those deer have been collected by the U.S. Department of Agriculture (USDA) for official CWD testing. MNPRO obtained additional research samples from the depopulated animals. Further testing of the carcass samples in-hand, as well as future collection and testing of additional samples from the carcass site, is dependent on MNPRO receiving additional funding.
The forensic recovery team included Larsen, Tiffany Wolf, DVM, Ph.D.; Roxanne Larsen, Ph.D.; Marc Schwabenlander, MPH; and Gage Rowden, M.S., all from the University of Minnesota College of Veterinary Medicine. Joining the team was Jason Bartz, Ph.D., from Creighton University’s School of Medicine. Bartz will independently verify the results of the RT-QuIC testing performed by the MNPRO laboratory.
The MNPRO team also recently developed a new assay that generates a color change of red for a positive CWD result and blue for negative. They have named the test “MN-QuIC” to honor the state of Minnesota, where the test was developed. The new test is cheaper than those using traditional equipment and uses field-deployable equipment to garner preliminary results in just 24 hours. The team is striving for a test that could be used at individual stations, cutting down on testing bottlenecks during deer hunting season. MN-QuIC is another tool that holds promise for rapid sample screening in forensic investigations such as this.
CWD originated roughly 50 years ago and affects white-tailed deer, mule deer, red deer, sika deer, caribou, reindeer, elk, and moose — all animals known as “cervids.” The disease produces small lesions in an animal’s brain and ultimately results in abnormal behavior, weight loss, loss of bodily functions, and death. While it is yet unknown whether the disease can spread to humans, the Centers for Disease Control and Prevention recommends against eating meat from CWD-infected animals. In 2020, both the Food and Drug Administration and the USDA declared CWD-positive venison unfit for human or animal consumption.
CWD is spread by misfolded prion proteins, the same process that causes scrapie in sheep, bovine spongiform encephalopathy in cattle (sometimes called “mad cow disease”), and sporadic Creutzfeldt-Jakob disease in humans. CWD-causing prions are not alive and can only be destroyed with specialized equipment or strong chemicals, which is what makes CWD so difficult to mitigate. They can also persist in the environment for years. Advances made on CWD could inform other prion-related diseases in humans and animals alike.
MNPRO’s research is supported by the MN Agricultural Experiment Station Rapid Ag Response Fund and the Minnesota Environment and Natural Resources Trust Fund, as recommended by the Legislative-Citizen Commission on Minnesota Resources. Additionally, various entities at the University of Minnesota have provided support, including the University’s Department of Veterinary and Biomedical Sciences, the Office for the Vice President of Research, and the College of Veterinary Medicine.
Categories: Science and Technology Animals Science
WEDNESDAY, APRIL 07, 2021
Minnesota 3-year-old white-tailed doe at a Beltrami County farm has been confirmed CWD positive
Should Property Evaluations Contain Scrapie, CWD, TSE PRION Environmental Contamination of the land ?
Scrapie, CWD, TSE PRION Environmental Contamination
***> For what it's worth, Back around 2000, 2001, or so, I was corresponding with officials abroad during the bse inquiry, passing info back and forth on CJD and Nutritional Supplements and BSE here in the USA, and some officials from here inside USDA aphis FSIS et al, in fact helped me get into the USA 50 state emergency BSE conference call way back. That one was a doozy. But I always remember what “deep throat” as i called them, I never knew who they were, but I never forgot what i was told decades ago, amongst them was ;
Some unofficial information from a source on the inside looking out -
***> Confidential!!!!
***> As early as 1992-3 there had been long studies conducted on small pastures containing scrapie infected sheep at the sheep research station associated with the Neuropathogenesis Unit in Edinburgh, Scotland. Whether these are documented...I don't know. But personal recounts both heard and recorded in a daily journal indicate that leaving the pastures free and replacing the topsoil completely at least 2 feet of thickness each year for SEVEN years....and then when very clean (proven scrapie free) sheep were placed on these small pastures.... the new sheep also broke out with scrapie and passed it to offspring. I am not sure that TSE contaminated ground could ever be free of the agent!! A very frightening revelation!!!
---end personal email---end...tss
and so it seems ;
***> This is very likely to have parallels with control efforts for CWD in cervids.
Paper
Rapid recontamination of a farm building occurs after attempted prion removal
Kevin Christopher Gough BSc (Hons), PhD Claire Alison Baker BSc (Hons) Steve Hawkins MIBiol Hugh Simmons BVSc, MRCVS, MBA, MA Timm Konold DrMedVet, PhD, MRCVS … See all authors
First published: 19 January 2019 https://doi.org/10.1136/vr.105054
Abstract
The transmissible spongiform encephalopathy scrapie of sheep/goats and chronic wasting disease of cervids are associated with environmental reservoirs of infectivity. Preventing environmental prions acting as a source of infectivity to healthy animals is of major concern to farms that have had outbreaks of scrapie and also to the health management of wild and farmed cervids. Here, an efficient scrapie decontamination protocol was applied to a farm with high levels of environmental contamination with the scrapie agent. Post‐decontamination, no prion material was detected within samples taken from the farm buildings as determined using a sensitive in vitro replication assay (sPMCA). A bioassay consisting of 25 newborn lambs of highly susceptible prion protein genotype VRQ/VRQ introduced into this decontaminated barn was carried out in addition to sampling and analysis of dust samples that were collected during the bioassay. Twenty‐four of the animals examined by immunohistochemical analysis of lymphatic tissues were scrapie‐positive during the bioassay, samples of dust collected within the barn were positive by month 3. The data illustrates the difficulty in decontaminating farm buildings from scrapie, and demonstrates the likely contribution of farm dust to the recontamination of these environments to levels that are capable of causing disease.
snip...
This study clearly demonstrates the difficulty in removing scrapie infectivity from the farm environment. Practical and effective prion decontamination methods are still urgently required for decontamination of scrapie infectivity from farms that have had cases of scrapie and this is particularly relevant for scrapiepositive goatherds, which currently have limited genetic resistance to scrapie within commercial breeds.24 This is very likely to have parallels with control efforts for CWD in cervids.
snip...see full text;
2021 Transmissible Spongiform Encephalopathy TSE Prion End of Year Report 2020
CJD FOUNDATION VIRTUAL CONFERENCE CJD Foundation Research Grant Recipient Reports Panel 2 Nov 3, 2020
zoonotic potential of PMCA-adapted CWD PrP 96SS inoculum
4 different CWD strains, and these 4 strains have different potential to induce any folding of the human prion protein.
***> PIGS, WILD BOAR, CWD <***
***> POPULATIONS OF WILD BOARS IN THE UNITED STATES INCREASING SUPSTANTUALLY AND IN MANY AREAS WE CAN SEE A HIGH DENSITY OF WILD BOARS AND HIGH INCIDENT OF CHRONIC WASTING DISEASE
HYPOTHOSIS AND SPECIFIC AIMS
HYPOTHOSIS
BSE, SCRAPIE, AND CWD, EXPOSED DOMESTIC PIGS ACCUMULATE DIFFERENT QUANTITIES AND STRAINS OF PRIONS IN PERIPHERAL TISSUES, EACH ONE OF THEM WITH PARTICULAR ZOONOTIC POTENTIALS
Final Report – CJD Foundation Grant Program A.
Project Title: Systematic evaluation of the zoonotic potential of different CWD isolates. Principal Investigator: Rodrigo Morales, PhD.
Systematic evaluation of the zoonotic potential of different CWD isolates. Rodrigo Morales, PhD Assistant Professor Protein Misfolding Disorders lab Mitchell Center for Alzheimer’s disease and Related Brain Disorders Department of Neurology University of Texas Health Science Center at Houston Washington DC. July 14th, 2018
Conclusions and Future Directions • We have developed a highly sensitive and specific CWD-PMCA platform to be used as a diagnostic tool. • Current PMCA set up allow us to mimic relevant prion inter-species transmission events. • Polymorphic changes at position 96 of the prion protein apparently alter strain properties and, consequently, the zoonotic potential of CWD isolates. • Inter-species and inter-polymorphic PrPC → PrPSc conversions further increase the spectrum of CWD isolates possibly present in nature. • CWD prions generated in 96SS PrPC substrate apparently have greater inter-species transmission potentials. • Future experiments will explore the zoonotic potential of CWD prions along different adaptation scenarios, including inter-species and inter-polymorphic.
Research Project: TRANSMISSION, DIFFERENTIATION, AND PATHOBIOLOGY OF TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES Location: Virus and Prion Research
Title: Disease-associated prion protein detected in lymphoid tissues from pigs challenged with the agent of chronic wasting disease
Author item MOORE, SARAH - Orise Fellow item Kunkle, Robert item KONDRU, NAVEEN - Iowa State University item MANNE, SIREESHA - Iowa State University item SMITH, JODI - Iowa State University item KANTHASAMY, ANUMANTHA - Iowa State University item WEST GREENLEE, M - Iowa State University item Greenlee, Justin Submitted to: Prion Publication Type: Abstract Only Publication Acceptance Date: 3/15/2017 Publication Date: N/A Citation: N/A Interpretive Summary:
Technical Abstract: Aims: Chronic wasting disease (CWD) is a naturally-occurring, fatal neurodegenerative disease of cervids. We previously demonstrated that disease-associated prion protein (PrPSc) can be detected in the brain and retina from pigs challenged intracranially or orally with the CWD agent. In that study, neurological signs consistent with prion disease were observed only in one pig: an intracranially challenged pig that was euthanized at 64 months post-challenge. The purpose of this study was to use an antigen-capture immunoassay (EIA) and real-time quaking-induced conversion (QuIC) to determine whether PrPSc is present in lymphoid tissues from pigs challenged with the CWD agent.
Methods: At two months of age, crossbred pigs were challenged by the intracranial route (n=20), oral route (n=19), or were left unchallenged (n=9). At approximately 6 months of age, the time at which commercial pigs reach market weight, half of the pigs in each group were culled (<6 month challenge groups). The remaining pigs (>6 month challenge groups) were allowed to incubate for up to 73 months post challenge (mpc). The retropharyngeal lymph node (RPLN) was screened for the presence of PrPSc by EIA and immunohistochemistry (IHC). The RPLN, palatine tonsil, and mesenteric lymph node (MLN) from 6-7 pigs per challenge group were also tested using EIA and QuIC.
Results: PrPSc was not detected by EIA and IHC in any RPLNs. All tonsils and MLNs were negative by IHC, though the MLN from one pig in the oral <6 month group was positive by EIA. PrPSc was detected by QuIC in at least one of the lymphoid tissues examined in 5/6 pigs in the intracranial <6 months group, 6/7 intracranial >6 months group, 5/6 pigs in the oral <6 months group, and 4/6 oral >6 months group. Overall, the MLN was positive in 14/19 (74%) of samples examined, the RPLN in 8/18 (44%), and the tonsil in 10/25 (40%).
Conclusions: This study demonstrates that PrPSc accumulates in lymphoid tissues from pigs challenged intracranially or orally with the CWD agent, and can be detected as early as 4 months after challenge. CWD-infected pigs rarely develop clinical disease and if they do, they do so after a long incubation period. This raises the possibility that CWD-infected pigs could shed prions into their environment long before they develop clinical disease. Furthermore, lymphoid tissues from CWD-infected pigs could present a potential source of CWD infectivity in the animal and human food chains.
Research Project: Pathobiology, Genetics, and Detection of Transmissible Spongiform Encephalopathies Location: Virus and Prion Research
Title: The agent of chronic wasting disease from pigs is infectious in transgenic mice expressing human PRNP
Author item MOORE, S - Orise Fellow item Kokemuller, Robyn item WEST-GREENLEE, M - Iowa State University item BALKEMA-BUSCHMANN, ANNE - Friedrich-Loeffler-institut item GROSCHUP, MARTIN - Friedrich-Loeffler-institut item Greenlee, Justin Submitted to: Prion Publication Type: Abstract Only Publication Acceptance Date: 5/10/2018 Publication Date: 5/22/2018 Citation: Moore, S.J., Kokemuller, R.D., West-Greenlee, M.H., Balkema-Buschmann, A., Groschup, M.H., Greenlee, J.J. 2018. The agent of chronic wasting disease from pigs is infectious in transgenic mice expressing human PRNP. Prion 2018, Santiago de Compostela, Spain, May 22-25, 2018. Paper No. WA15, page 44.
Interpretive Summary:
Technical Abstract: We have previously shown that the chronic wasting disease (CWD) agent from white-tailed deer can be transmitted to domestic pigs via intracranial or oral inoculation although with low attack rates and restricted PrPSc accumulation. The objective of this study was to assess the potential for cross-species transmission of pig-passaged CWD using bioassay in transgenic mice. Transgenic mice expressing human (Tg40), bovine (TgBovXV) or porcine (Tg002) PRNP were inoculated intracranially with 1% brain homogenate from a pig that had been intracranially inoculated with a pool of CWD from white-tailed deer. This pig developed neurological clinical signs, was euthanized at 64 months post-inoculation, and PrPSc was detected in the brain. Mice were monitored daily for clinical signs of disease until the end of the study. Mice were considered positive if PrPSc was detected in the brain using an enzyme immunoassay (EIA). In transgenic mice expressing porcine prion protein the average incubation period was 167 days post-inoculation (dpi) and 3/27 mice were EIA positive (attack rate = 11%). All 3 mice were found dead and clinical signs were not noted prior to death. One transgenic mouse expressing bovine prion protein was euthanized due to excessive scratching at 617 dpi and 2 mice culled at the end of the study at 700 dpi were EIA positive resulting in an overall attack rate of 3/16 (19%). None of the transgenic mice expressing human prion protein that died or were euthanized up to 769 dpi were EIA positive and at study end point at 800 dpi 2 mice had positive EIA results (overall attack rate = 2/20 = 10%). The EIA optical density (OD) readings for all positive mice were at the lower end of the reference range (positive mice range, OD = 0.266-0.438; test positive reference range, OD = 0.250-4.000). To the authors’ knowledge, cervid-derived CWD isolates have not been successfully transmitted to transgenic mice expressing human prion protein. The successful transmission of pig-passaged CWD to Tg40 mice reported here suggests that passage of the CWD agent through pigs results in a change of the transmission characteristics which reduces the transmission barrier of Tg40 mice to the CWD agent.
If this biological behavior is recapitulated in the original host species, passage of the CWD agent through pigs could potentially lead to increased pathogenicity of the CWD agent in humans.
cwd scrapie pigs oral routes
***> However, at 51 months of incubation or greater, 5 animals were positive by one or more diagnostic methods. Furthermore, positive bioassay results were obtained from all inoculated groups (oral and intracranial; market weight and end of study) suggesting that swine are potential hosts for the agent of scrapie. <***
>*** Although the current U.S. feed ban is based on keeping tissues from TSE infected cattle from contaminating animal feed, swine rations in the U.S. could contain animal derived components including materials from scrapie infected sheep and goats. These results indicating the susceptibility of pigs to sheep scrapie, coupled with the limitations of the current feed ban, indicates that a revision of the feed ban may be necessary to protect swine production and potentially human health. <***
***> Results: PrPSc was not detected by EIA and IHC in any RPLNs. All tonsils and MLNs were negative by IHC, though the MLN from one pig in the oral <6 month group was positive by EIA. PrPSc was detected by QuIC in at least one of the lymphoid tissues examined in 5/6 pigs in the intracranial <6 months group, 6/7 intracranial >6 months group, 5/6 pigs in the oral <6 months group, and 4/6 oral >6 months group. Overall, the MLN was positive in 14/19 (74%) of samples examined, the RPLN in 8/18 (44%), and the tonsil in 10/25 (40%).
***> Conclusions: This study demonstrates that PrPSc accumulates in lymphoid tissues from pigs challenged intracranially or orally with the CWD agent, and can be detected as early as 4 months after challenge. CWD-infected pigs rarely develop clinical disease and if they do, they do so after a long incubation period. This raises the possibility that CWD-infected pigs could shed prions into their environment long before they develop clinical disease. Furthermore, lymphoid tissues from CWD-infected pigs could present a potential source of CWD infectivity in the animal and human food chains.
America BSE 589.2001 FEED REGULATIONS, BSE SURVEILLANCE, BSE TESTING, and CJD TSE Prion
so far, we have been lucky. to date, with the science at hand, no cwd transmitted to cattle, that has been documented, TO DATE, WITH THE SCIENCE AT HAND, it's not to say it has not already happened, just like with zoonosis of cwd i.e. molecular transmission studies have shown that cwd transmission to humans would look like sporadic cjd, NOT nvCJD or what they call now vCJD. the other thing is virulence and or horizontal transmission. this is very concerning with the recent fact of what seems to be a large outbreak of a new tse prion disease in camels in Africa. there is much concern now with hay, straw, grains, and such, with the cwd tse prion endemic countries USA, Canada. what is of greatest concern is the different strains of cwd, and the virulence there from? this thing (cwd) keeps mutating to different strains, and to different species, the bigger the chance of one of these strains that WILL TRANSMIT TO CATTLE OR HUMANS, and that it is documented (i believe both has already occured imo with scienct to date). with that said, a few things to ponder, and i am still very concerned with, the animal feed. we now know from transmission studies that cwd and scrapie will transmit to pigs by oral routes. the atypical bse strains will transmit by oral routes. i don't mean to keep kicking a mad cow, just look at the science;
***> cattle, pigs, sheep, cwd, tse, prion, oh my!
***> In contrast, cattle are highly susceptible to white-tailed deer CWD and mule deer CWD in experimental conditions but no natural CWD infections in cattle have been reported (Sigurdson, 2008; Hamir et al., 2006).
Sheep and cattle may be exposed to CWD via common grazing areas with affected deer but so far, appear to be poorly susceptible to mule deer CWD (Sigurdson, 2008). In contrast, cattle are highly susceptible to white-tailed deer CWD and mule deer CWD in experimental conditions but no natural CWD infections in cattle have been reported (Sigurdson, 2008; Hamir et al., 2006). It is not known how susceptible humans are to CWD but given that the prion can be present in muscle, it is likely that humans have been exposed to the agent via consumption of venison (Sigurdson, 2008). Initial experimental research suggests that human susceptibility to CWD is low and there may be a robust species barrier for CWD transmission to humans (Sigurdson, 2008), however the risk appetite for a public health threat may still find this level unacceptable.
Friday, December 14, 2012
DEFRA U.K. What is the risk of Chronic Wasting Disease CWD being introduced into Great Britain? A Qualitative Risk Assessment October 2012
snip.....
In the USA, under the Food and Drug Administration's BSE Feed Regulation (21 CFR 589.2000) most material (exceptions include milk, tallow, and gelatin) from deer and elk is prohibited for use in feed for ruminant animals. With regards to feed for non-ruminant animals, under FDA law, CWD positive deer may not be used for any animal feed or feed ingredients. For elk and deer considered at high risk for CWD, the FDA recommends that these animals do not enter the animal feed system. However, this recommendation is guidance and not a requirement by law. Animals considered at high risk for CWD include:
1) animals from areas declared to be endemic for CWD and/or to be CWD eradication zones and
2) deer and elk that at some time during the 60-month period prior to slaughter were in a captive herd that contained a CWD-positive animal.
Therefore, in the USA, materials from cervids other than CWD positive animals may be used in animal feed and feed ingredients for non-ruminants.
The amount of animal PAP that is of deer and/or elk origin imported from the USA to GB can not be determined, however, as it is not specified in TRACES.
It may constitute a small percentage of the 8412 kilos of non-fish origin processed animal proteins that were imported from US into GB in 2011.
Overall, therefore, it is considered there is a __greater than negligible risk___ that (nonruminant) animal feed and pet food containing deer and/or elk protein is imported into GB.
There is uncertainty associated with this estimate given the lack of data on the amount of deer and/or elk protein possibly being imported in these products.
snip.....
36% in 2007 (Almberg et al., 2011). In such areas, population declines of deer of up to 30 to 50% have been observed (Almberg et al., 2011). In areas of Colorado, the prevalence can be as high as 30% (EFSA, 2011). The clinical signs of CWD in affected adults are weight loss and behavioural changes that can span weeks or months (Williams, 2005). In addition, signs might include excessive salivation, behavioural alterations including a fixed stare and changes in interaction with other animals in the herd, and an altered stance (Williams, 2005). These signs are indistinguishable from cervids experimentally infected with bovine spongiform encephalopathy (BSE). Given this, if CWD was to be introduced into countries with BSE such as GB, for example, infected deer populations would need to be tested to differentiate if they were infected with CWD or BSE to minimise the risk of BSE entering the human food-chain via affected venison. snip..... The rate of transmission of CWD has been reported to be as high as 30% and can approach 100% among captive animals in endemic areas (Safar et al., 2008).
snip.....
In summary, in endemic areas, there is a medium probability that the soil and surrounding environment is contaminated with CWD prions and in a bioavailable form. In rural areas where CWD has not been reported and deer are present, there is a greater than negligible risk the soil is contaminated with CWD prion. snip..... In summary, given the volume of tourists, hunters and servicemen moving between GB and North America, the probability of at least one person travelling to/from a CWD affected area and, in doing so, contaminating their clothing, footwear and/or equipment prior to arriving in GB is greater than negligible... For deer hunters, specifically, the risk is likely to be greater given the increased contact with deer and their environment. However, there is significant uncertainty associated with these estimates.
snip.....
Therefore, it is considered that farmed and park deer may have a higher probability of exposure to CWD transferred to the environment than wild deer given the restricted habitat range and higher frequency of contact with tourists and returning GB residents.
snip.....
Research Project: Pathobiology, Genetics, and Detection of Transmissible Spongiform Encephalopathies Location: Virus and Prion Research
Title: Raccoons accumulate PrPSc after intracranial inoculation with the agents of chronic wasting disease (CWD) or transmissible mink encephalopathy (TME) but not atypical scrapie
Author item MOORE, S - Orise Fellow item Smith, Jodi item Richt, Juergen item Greenlee, Justin Submitted to: Journal of Veterinary Diagnostic Investigation Publication Type: Peer Reviewed Journal Publication Acceptance Date: 12/22/2018 Publication Date: 1/29/2019 Citation: Moore, S.J., Smith, J.D., Richt, J., Greenlee, J.J. 2019. Raccoons accumulate PrPSc after intracranial inoculation with the agents of chronic wasting disease (CWD) or transmissible mink encephalopathy (TME) but not atypical scrapie. Journal of Veterinary Diagnostic Investigation. 31(2):200-209. https://doi.org/10.1177/1040638718825290. DOI: https://doi.org/10.1177/1040638718825290 Interpretive Summary: The prion diseases are fatal diseases of animals and humans that cause damaging changes in the brain. Animal prion diseases include scrapie in sheep, chronic wasting disease (CWD) in cervids, and transmissible mink encephalopathy (TME) in ranch-raised mink. The infectious agent is an abnormal protein called a prion that has misfolded from its normal state. This study tested whether raccoons develop clinical disease and/or accumulate abnormal prion protein after inoculation with prion agents from different species: TME from cattle, raccoons, or hamsters that occurs in two forms with distinct clinical signs and molecular properties called hyper and drowsy; CWD from white-tailed deer or elk; and atypical (Nor-98) scrapie from sheep. All raccoons inoculated with TME from raccoons or cattle developed clinical disease with short survival times. Raccoons inoculated with CWD from white-tailed deer, CWD from elk, or 'hyper' TME from hamsters did not develop clinical disease, but abnormal prion protein was detected in the brains of 25% of the raccoons in each study. The amount of abnormal prion protein in the brains of these raccoons was much less than in the brains of raccoons inoculated with TME from raccoons or cattle. None of the raccoons inoculated with 'drowsy' TME from hamsters or atypical scrapie from sheep developed clinical disease or detectable abnormal prion protein. This work suggests that raccoons are susceptible to prion disease isolates from raccoons, cattle, white-tailed deer, and elk. Raccoons are omnivores that have a widespread geographical distribution and are known to scavenge animal carcasses. Therefore, they could provide a route of transmission of prions disease between farmed and wild animal species. This information is useful to farmers and people involved in control of prion disease in free-ranging animals.
Technical Abstract: The prion diseases are neurodegenerative diseases characterized by the accumulation of misfolded prion protein (PrP*Sc) in the brain and other tissues. Animal prion diseases include scrapie in sheep, chronic wasting disease (CWD) in cervids, and transmissible mink encephalopathy (TME) in ranch-raised mink. The objective of this study was to investigate the susceptibility of raccoons to various prion disease isolates, and to compare the clinicopathologic features of the resulting disease. Raccoon kits were inoculated intracranially with raccoon-passaged TME (TME*Rac), bovine-passaged TME (TME*Bov), hamster-adapted drowsy (TME*DY) or hyper TME (TME*HY), CWD from white-tailed deer (CWD*Wtd) or elk (CWD*Elk), or atypical (Nor-98) scrapie. Raccoons were euthanized when they developed clinical signs of prion disease or at study endpoint (<82 months post-inoculation). Brain was examined for the presence of spongiform change and disease-associated PrP*Sc was detected using an enzyme-linked immunoassay, western immunoblot, and immunohistochemistry. All raccoons inoculated with TME*Rac and TME*Bov developed clinical disease at around 6.6 months post-inoculation with widespread PrP*Sc accumulation in central nervous system tissues. PrP*Sc was detected in the brain from 1 out of 4 raccoons in each of the CWD*Wtd, CWD*Elk, and TME*HY inoculated groups. None of the raccoons inoculated with TME*DY or atypical scrapie developed clinical disease or detectable PrP*Sc accumulation. The results of this study indicate that raccoons are highly susceptible to infection with raccoon- and bovine-passaged TME, while CWD isolates from white-tailed deer or elk and hamster-adapted TME*HY transmit poorly. Raccoons appear to be resistant to infection with hamster-adapted TME*DY and atypical scrapie.
Rabbits are not resistant to prion infection
Francesca Chianinia,1, Natalia Fernández-Borgesb,c,1, Enric Vidald , Louise Gibbarda , Belén Pintadoe , Jorge de Castroc , Suzette A. Priolaf , Scott Hamiltona , Samantha L. Eatona , Jeanie Finlaysona , Yvonne Panga , Philip Steelea , Hugh W. Reida , Mark P. Dagleisha , and Joaquín Castillab,c,g,2 a Moredun Research Institute, Penicuik, Near Edinburgh EH26 0PZ, Scotland, United Kingdom; b CIC bioGUNE, Derio 48160, Bizkaia, Spain; g IKERBASQUE, Basque Foundation for Science, Bilbao 48011, Bizkaia, Spain; c Department of Infectology, Scripps Florida, Jupiter, FL 33458; f Laboratory of Persistent Viral Diseases, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840; d Centre de Recerca en Sanitat Animal (CReSA), UAB-IRTA, Universitat Autònoma de Barcelona, 08193 Bellaterra, Barcelona, Spain; and e Centro Nacional de Biotecnología (CNB), 28049 Cantoblanco, Madrid, Spain Edited by Reed B. Wickner, National Institutes of Health, Bethesda, MD, and approved February 16, 2012 (received for review December 6, 2011)
The ability of prions to infect some species and not others is determined by the transmission barrier. This unexplained phenomenon has led to the belief that certain species were not susceptible to transmissible spongiform encephalopathies (TSEs) and therefore represented negligible risk to human health if consumed. Using the protein misfolding cyclic amplification (PMCA) technique, we were able to overcome the species barrier in rabbits, which have been classified as TSE resistant for four decades. Rabbit brain homogenate, either unseeded or seeded in vitro with disease-related prions obtained from different species, was subjected to serial rounds of PMCA. De novo rabbit prions produced in vitro from unseeded material were tested for infectivity in rabbits, with one of three intracerebrally challenged animals succumbing to disease at 766 d and displaying all of the characteristics of a TSE, thereby demonstrating that leporids are not resistant to prion infection. Material from the brain of the clinically affected rabbit containing abnormal prion protein resulted in a 100% attack rate after its inoculation in transgenic mice overexpressing rabbit PrP. Transmissibility to rabbits (>470 d) has been confirmed in 2 of 10 rabbits after intracerebral challenge. Despite rabbits no longer being able to be classified as resistant to TSEs, an outbreak of “mad rabbit disease” is unlikely.
snip...
To critically evaluate this risk, several experiments are currently underway to characterize this new prion disease in rabbits and other species to examine its ability to cross the species barrier. In addition, supplementary experiments have been initiated in rabbits and also in transgenic mice that overexpress rabbit PrPC, to evaluate their susceptibilities to other important prion diseases including CWD and BSE. There are several factors that any potential new TSE epidemic would require: (i) the new prion should be efficiently transmitted through the homologous species; (ii) animals should be edible by humans and should be slaughtered at an age at which the disease has developed, thereby increasing the chance that prions have replicated (especially for those prions that require long incubation times); and (iii) the meat and bone meal should be recycled and fed to new members of the same species. In the light of these data and taking into account the previous three factors, it is unlikely there will be an outbreak of “mad rabbit disease,” and consumers of rabbit meat face much less of a risk than consumers of cattle or sheep products.
''it is unlikely there will be an outbreak of mad rabbit disease”
TELL THAT TO THE MAD CAMELS FROM THE NEW TSE PRION DISEASE OUTBREAK IN A NEW LIVESTOCK SPECIES IN AFRICA I.E. CAMEL PRION DISEASE...terry
Tuesday, April 27, 2021
Working Document on Camel Prion Disease (CPrD) 14/09/2020
Update on chronic wasting disease (CWD) III
EFSA Panel on Biological Hazards (BIOHAZ)
First published:11 November 2019 http:// https://doi.org/10.2903/j.efsa.2019.5863
Correspondence: biohaz@efsa.europa.eu
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00763
Adopted: 26 September 2019
This article was originally published on the EFSA website www.efsa.europa.eu on 7 November 2019
SNIP...
***> Movement of other animals (working dogs, scavengers, predators),
SNIP...
Whether the natural host range of CWD in North America extends beyond the family Cervidae is currently unclear and no natural infections have been reported so far in wildlife species with substantial overlapping geographical range and which could play a role in the spread of CWD, such as predators and scavengers.
snip...
A recent study investigated the potential for swine to serve as hosts of the CWD agent(s) by intracerebral or oral challenge of crossbred piglets (Moore et al., 2016b, 2017). Pigs sacrificed at 6 mpi, approximately the age at which pigs reach market weight, were clinically healthy and negative by diagnostic tests, although low‐level CWD agent replication could be detected in the CNS by bioassay in tg cervinised mice. Among pigs that were incubated for up to 73 mpi, some gave diagnostic evidence of CWD replication in the brain between 42 and 72 mpi. Importantly, this was observed also in one orally challenged pig at 64 mpi and the presence of low‐level CWD replication was confirmed by mouse bioassay. The authors of this study argued that pigs can support low‐level amplification of CWD prions, although the species barrier to CWD infection is relatively high and that the detection of infectivity in orally inoculated pigs with a mouse bioassay raises the possibility that naturally exposed pigs could act as a reservoir of CWD infectivity.
3.2.1.3 Other species
Studies have demonstrated that the CWD agent(s) can be transmitted by the IC route in several species of rodents, such as voles (Subfamily Arvicolinae), deer mice (Peromyscus maniculatus), mice and hamsters (Subfamily Cricetinae). The susceptibility was, however, variable, being high in voles and deer mice but lower in mice and hamsters (Raymond et al., 2007; Heisey et al., 2010; Kurt et al., 2011; Di et al., 2013; Lee et al., 2013). Mink (subfamily Mustelinae) (Harrington et al., 2008), ferrets (Mustela putorius) (Bartz et al., 1998; Sigurdson et al., 2008) and cats (Mathiason et al., 2013) were susceptible to IC challenge with NA CWD sources, while CWD transmitted poorly to raccoons (Procyon lotor) by the IC route (Moore et al., 2019).
SNIP...
10. Movement of other animals (working dogs, scavengers, predators)
snip...
Scavengers Various species of (raptors, corvids) birds or mammals that feed on animal carcasses can act as spreaders of the infection. It has been shown that carcasses abandoned in the field in an area of Wisconsin were a source of food for at least 14 species of mammals and 14 species of birds. Carcasses could persist in the field from 18 to 101 days depending on the season and year. The involvement of the birds also suggests that the infectious agent could be transferred at great distances from the infected carcass (Jennelle et al., 2009) Jennelle et al. (2009) other F N Predators.
Prion‐infected deer were much more likely to be killed by mountain lions than uninfected deer (Miller et al., 2008). The presence of prions and their infectious ability in cervinised transgenic mice have been demonstrated in the faeces of coyotes (Canis latrans) 3 days after they had fed on with infected deer carcasses (Nichols et al., 2015). Faeces of predators (in North America e.g. coyotes or pumas) can serve as a vehicle for prions contributing to the spread of the infectious agent in the environment. Miller et al. (2008) cohort B N Nichols et al. (2015) other
***> 10. Movement of other animals (working dogs, scavengers, predators).
***> Faeces of predators (in North America e.g. coyotes or pumas) can serve as a vehicle for prions contributing to the spread of the infectious agent in the environment. Miller et al. (2008) cohort B N Nichols et al. (2015) other
SNIP...SEE FULL TEXT;
Prion. 2013 Jul 1; 7(4): 263–266.
Published online 2013 Jul 3. doi: 10.4161/pri.25621
PMCID: PMC3904308
PMID: 23822910
Could avian scavengers translocate infectious prions to disease-free areas initiating new foci of chronic wasting disease? Justin W Fischer, Gregory E Phillips, Tracy A Nichols, and Kurt C VerCauteren*
North American predators and scavengers, such as wolves (Canis lupus), mountain lions (Puma concolor), coyotes (Canis latrans), raccoons (Procyon lotor), opossums (Didelphis virginiana), vultures (Cathartes aura and Coragyps atratus), and crows (Corvus brachyrhynchos) may also participate in the spread of CWD. Jennelle et al., (2009) documented a host of mammals and birds that scavenged on white-tailed deer carcasses in central Wisconsin, with crows being a primary scavenger.15 These species could consume and transport infectious material through feces deposition,16,17 or simply transport of material through food-caching, young-provisioning, and other natural behaviors.
In conclusion, our study showed that the digestive system of crows did not eliminate PrPRes infectivity prior to excretion of feces,21 which suggests that avian scavengers may play a role in the transmission and translocation of prion diseases. Relatedly, crows often forage and defecate on feed at farmed cervid facilities, providing an opportunity for farmed cervids to ingest crow feces and crows to ingest feed with elk saliva, and other potentially PrPRes-infected material. Further experiments involving other avian, as well as mammalian, scavengers are needed to evaluate PrPRes infectivity after passage of natural transmissible spongiform encephalopathies through their digestive systems. We are currently conducting a study to evaluate CWD passage through the digestive system of coyotes. It would be prudent to evaluate other mammalian scavengers for their ability to act as intermediate CWD hosts between cervids and humans.
***> North American predators and scavengers, such as wolves (Canis lupus), mountain lions (Puma concolor), coyotes (Canis latrans), raccoons (Procyon lotor), opossums (Didelphis virginiana), vultures (Cathartes aura and Coragyps atratus), and crows (Corvus brachyrhynchos) may also participate in the spread of CWD. Jennelle et al., (2009) documented a host of mammals and birds that scavenged on white-tailed deer carcasses in central Wisconsin, with crows being a primary scavenger.15
1 July 2009
Deer Carcass Decomposition and Potential Scavenger Exposure to Chronic Wasting Disease
Christopher S. Jennelle, Michael D. Samuel, Cherrie A. Nolden, Elizabeth A. Berkley
Author Affiliations +
J. of Wildlife Management, 73(5):655-662 (2009). https://doi.org/10.2193/2008-282
Abstract
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy afflicting the Cervidae family in North America, causing neurodegeneration and ultimately death. Although there are no reports of natural cross-species transmission of CWD to noncervids, infected deer carcasses pose a potential risk of CWD exposure for other animals. We placed 40 disease-free white-tailed deer (Odocoileus virginianus) carcasses and 10 gut piles in the CWD-affected area of Wisconsin (USA) from September to April in 2003 through 2005. We used photos from remotely operated cameras to characterize scavenger visitation and relative activity. To evaluate factors driving the rate of carcass removal (decomposition), we used Kaplan–Meier survival analysis and a generalized linear mixed model. We recorded 14 species of scavenging mammals (6 visiting species) and 14 species of scavenging birds (8 visiting species). Prominent scavengers included American crows (Corvus brachyrhynchos), raccoons (Procyon lotor), and Virginia opossums (Didelphis virginiana). We found no evidence that deer consumed conspecific remains, although they visited gut piles more often than carcasses relative to temporal availability in the environment. Domestic dogs, cats, and cows either scavenged or visited carcass sites, which could lead to human exposure to CWD. Deer carcasses persisted for 18 days to 101 days depending on the season and year, whereas gut piles lasted for 3 days. Habitat did not influence carcass decomposition, but mammalian and avian scavenger activity and higher temperatures were positively associated with faster removal. Infected deer carcasses or gut piles can serve as potential sources of CWD prions to a variety of scavengers. In areas where surveillance for CWD exposure is practical, management agencies should consider strategies for testing primary scavengers of deer carcass material.
SPONGIFORM ENCEPHALOPATHY IN A CAPTIVE PUMA
an article in yesterday's Times (attached) which suggested that the puma concerned had never ''eaten any part of a cow or sheep which, in the opinion of Government Scientists, could transmit the species to a different species''.
3. You explained to me that this was INCORRECT. The position was as set out in the briefing for Prime Minister's questions attached to Mr Taylor's note. The puma had probably been fed low quality beef meat in the form of split carcasses. ...
Spongiform Encephalopathy in Captive Wild Animals in Britain
Sun, Dec 20, 2020 4:54 pm
Subject: TSE in exotic ruminants
TSE in exotic ruminants
NUMBER OF CONFIRMED CASES OF FSE IN DOMESTIC CATS BY YEAR Year Reported No. of cases Year of Onset No. of cases
1988 0 1988 0
1989 0 1989 1
1990 12 1990 16
1991 12 1991 11
1992 10 1992 14
1993 11 1993 10
1994 16 1994 14
1995 8 1995 4
1996 6 1996 7
1997 6 1997 8
1998 4 1998 1
1999 2 1999 1
2000 1 2000 1
2001 1 2001 1
2002 0 2002 0
2003 0 2003 0
2004 0 2004 0
2005 0 2005 0
2006 0 2006 0
2007 0 2007 0
2008 0 2008 0
2009 0 2009 0
2010 0 2010 0
2011 0 2011 0
2012 0 2012 0
2013 0 2013 0
2014 0 2014 0
2015 0 2015 0
2016 0 2016 0
2017 0 2017 0
2018 0 2018 0
2019 0 2019 0
2020 0 2020 0
Total 89 Total 89 Data valid to 30 November 2020 Includes one case from Guernsey
Published 11 February 2015 Last updated 21 December 2020 - hide all updates
SE DIAGNOSES IN EXOTIC SPECIES
KUDU 6
GEMSBOK 1
NYALA 1
ORYX 2
ELAND 6
CHEETAH 4*
PUMA 3
TIGER 1
OCELOT 2
BISON (bison bison) 1
ANKOLE COW 2
* Excludes one cheetah in Australia and one in ROI - litter mates born in GB, and another in France also born in G.B. [figures to 1 January 1998]
FELINE SPONGIFORM ENCEPHALOPATHY
TOTAL TO DATE 81 (Plus 1 in N Ireland, 1 in Norway, 1 in Lichtenstein )
YEAR Cases
1990 12
1991 12
1992 10
1993 11
1994 16
1995 8
1996 6
1997 6
Exotic species
Species Number of cases Dates affected
Ankole Cow 2 1991, 95
Bison 1 1996
Asian Leopard Cat (1) 1 2005
Cheetah 5 1992, 98
Eland 6 1989, 95
Gemsbok 1 1987
Kudu 6 1989, 92
Lion 4 1998, 2001
Nyala 1 1986
Ocelot 3 1994, 99
Oryx 2 1989, 92
Puma 3 1992, 95
Tiger 3 1995, 99
As at 12 January 2006.
A total of 38 cases of spongiform encephalopathy have been confirmed in exotic species, the last one in 2005.
(1) Felis (Prionailurus) bengalensis.
BSE TSE PRION STATISTICS
ZOO ANIMALS AND TSE PRION DISEASE
The 82 zoo animals with BSE:
Id TSE Genus Species Subsp Birth Origin Death Place of Death
654 x Microcebus murinus - 1997 U.Montpellier 1998 U.Montpellier
656 x Microcebus murinus - 1997 U.Montpellier 1998 U.Montpellier
481 + Eulemur fulvus mayottensis 1974 Madagascar 1992 Montpellier zoo
474 + Eulemur fulvus mayottensis 1974 Madagascar 1990 Montpellier zoo
584 - Eulemur fulvus mayottensis 1984 Montpellier 1991 Montpellier zoo
455 + Eulemur fulvus mayottensis 1983 Montpellier 1989 Montpellier zoo
- + Eulemur fulvus mayottensis 1988 Montpellier 1992 Montpellier zoo
- + Eulemur fulvus mayottensis 1995 Montpellier 1996 Montpellier zoo
- + Eulemur fulvus albifrons 1988 Paris 1992 Montpellier zoo
- + Eulemur fulvus albifrons 1988 Paris 1990 Montpellier zoo
- + Eulemur fulvus albifrons 1988 Paris 1992 Montpellier zoo
456 + Eulemur fulvus albifrons 1988 Paris 1990 Montpellier zoo
586 + Eulemur mongoz - 1979 Madagascar 1998 Montpellier zoo
- p Eulemur mongoz - 1989 Mulhouse 1991 Montpellier zoo
- p Eulemur mongoz - 1989 Mulhouse 1990 Montpellier zoo
- p Eulemur macaco - 1986 Montpellier 1996 Montpellier zoo
- p Lemur catta - 1976 Montpellier 1994 Montpellier zoo
- p Varecia variegata variegata 1985 Mulhouse 1990 Montpellier zoo
- p Varecia variegata variegata 1993 xxx 1994 Montpellier zoo
455 + Macaca mulatta - 1986 Ravensden UK 1992 Montpellier zoo
- p Macaca mulatta - 1986 Ravensden UK 1993 Montpellier zoo
- p Macaca mulatta - 1988 Ravensden UK 1991 Montpellier zoo
- p Saimiri sciureus - 1987 Frejus France 1990 Frejus zoo
700 pc eulemur hybrid - - Besancon zoo 1998 Besancon zoo
701 pc eulemur hybrid - - Besancon zoo 1998 Besancon zoo
702 pc eulemur hybrid - - Besancon zoo 1998 Besancon zoo
703 pc eulemur hybrid - - Besancon zoo 1998 Besancon zoo
704 pc eulemur hybrid - - Besancon zoo 1998 Besancon zoo
705 pc eulemur hybrid - - Besancon zoo 1998 Besancon zoo
706 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
707 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
708 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
709 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
710 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
711 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
712 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
713 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
714 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
715 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
716 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
717 pc eulemur hybrid - - Strasbourg zoo 1998 Strasbourg zoo
x p genus species - - Lille zoo 1996 Lille zoo
y p genus species - - Lille zoo 1996 Lille zoo
z p genus species - - Lille zoo 1996 Lille zoo
1 + Actinonyx jubatus cheetah 1986 Marwell zoo 1991 Pearle Coast AU
Duke + Actinonyx jubatus cheetah 1984 Marwell zoo 1992 Colchester zoo? UK
Saki + Actinonyx jubatus cheetah 1986 Marwell zoo 1993 unknown UK
Mich + Actinonyx jubatus cheetah 1986 Whipsnade 1993 Whipsnade UK
Fr1 + Actinonyx jubatus cheetah 1987 Whipsnade 1997 Safari de Peaugres FR
Fr2 + Actinonyx jubatus cheetah 1991 Marwell zoo 1997 Safari de Peaugres Fr
xx + Actinonyx jubatus cheetah 19xx xxx zoo 199x Fota zoo IR
yy + Actinonyx jubatus cheetah 19xx yyy zoo 1996+ yyyy zoo UK
zz + Actinonyx jubatus cheetah 19xx zzz zoo 1996+ yyyy zoo UK
aaa + Felis concolor puma 1986 Chester zoo 1991 Chester zoo UK
yy + Felis concolor puma 1980 yyy zoo 1995 yyyy zoo UK
zz + Felis concolor puma 1978 zzz zoo 1995 zzzz zoo UK
xxx + Felis pardalis ocelot 1987 xxx 1994 Chester zoo UK
zzz + Felis pardalis ocelot 1980 zzz 1995 zzzz zoo UK
85 + Felis catus cat 1990+ various 1999+ various UK LI NO
19 + Canis familia. dog 1992+ various 1999+ various UK
Fota + Panthera tigris tiger 1981 xxx zoo 1995 xxxx zoo UK
yy + Panthera tigris tiger 1983 yyy zoo 1998 yyyy zoo UK
Lump + Panthera leo lion 1986 Woburn SP 1998 Edinburgh zoo UK [since 1994]
1 + Taurotragus oryx eland 1987 Port Lympne 1989 Port Lympne zoo UK
Moll + Taurotragus oryx eland 1989 xx UK 1991 not Port Lympne UK
Nedd + Taurotragus oryx eland 1989 xx UK 1991 not Port Lympne UK
Elec + Taurotragus oryx eland 1990 xx UK 1992 not Port Lympne Uk
Daph p Taurotragus oryx eland 1988 xx UK 1990 not Port Lympne UK
zzz + Taurotragus oryx eland 1991 zz UK 1994 zzz UK
yyy + Taurotragus oryx eland 1993 yy UK 1995 yyy UK
Fran p Tragelaphus strepsi. kudu 1985 London zoo 1987 London zoo UK
Lind + Tragelaphus strepsi. kudu 1987 London zoo 1989 London zoo UK
Karl + Tragelaphus strepsi. kudu 1988 London zoo 1990 London zoo UK
Kaz + Tragelaphus strepsi. kudu 1988 London zoo 1991 London zoo UK
Bamb pc Tragelaphus strepsi. kudu 1988 London zoo 1991 London zoo UK
Step - Tragelaphus strepsi. kudu 1984 London zoo 1991 London zoo UK
346 pc Tragelaphus strepsi. kudu 1990 London zoo 1992 London zoo UK
324 + Tragelaphus strepsi. kudu 1989 Marwell zoo 1992 London zoo UK
xxx + Tragelaphus angasi nyala 1983 Marwell zoo 1986 Marwell zoo UK
yy + Oryx gazella gemsbok 1983 Marwell zoo 1986 Marwell zoo UK
zz + Oryx gazella gemsbok 1994+ zzz zoo 1996+ zzzz zoo UK
xx + Oryx dammah scim oryx 1990 xxxx zoo 1993 Chester zoo UK
yy + Oryx leucoryx arab oryx 1986 Zurich zoo 1991 London zoo UK
yy + Bos taurus ankole cow 1987 yyy zoo 1995 yyyy zoo UK
zz + Bos taurus ankole cow 1986 zzz zoo 1991 zzzz zoo UK
xx + Bison bison Eu bison 1989 xxx zoo 1996 xxxx zoo UK
Vet Rec 1997 Sep 13;141(11):270-1 Baron-T, Belli-P Madec-J-Y Moutou-F Vitaud-C Savey-M Spongiform encephalopathy in an imported cheetah in France. CNEVA-Lyon, Laboratoire de Pathologie Bovine, France.
Proc Soc Exp Biol Med 1996 Apr;211(4):306-22 Narang H Origin and implications of bovine spongiform encephalopathy. [tiger]
Vet Rec. 1994 Nov 12;135(20):488. Benbow G. Spongiform encephalopathies in zoo animals. comment
Vet Rec 1994 Oct 29;135(18):440 Swainston J. comment
Vet Rec 1994 Sep 24;135(13):296-303 Kirkwood JK, Cunningham AA Epidemiological observations on spongiform encephalopathies
Vet Rec 1994 Feb 12;134(7):167-8 Kirkwood JK, Cunningham AA, Austin AR, Wells GA, Sainsbury AW Spongiform encephalopathy in a greater kudu
Vet Rec. 1993 Oct 9;133(15):360-4. Kirkwood JK, et al. Spongiform encephalopathy in a herd of greater kudu
Vet Rec. 1993 Jan 16;132(3):68. Cunningham AA, et al. Transmissible spongiform encephalopathy in greater kudu
Vet Rec. 1992 Nov 7;131(19):431-4. Willoughby K, et al. Spongiform encephalopathy in a captive puma
Aust Vet J 1992 Jul;69(7):171 Peet RL, Curran JM Spongiform encephalopathy in an imported cheetah
Vet Rec 1992 Apr 25;130(17):365-7 Kirkwood JK, Wells GA, Cunningham AA, Jackson SI, Scott AC, Dawson M, Wilesmith JW Scrapie-like encephalopathy in a greater kudu
Acta Neuropathol (Berl) 1992;84(5):559-69 Jeffrey M, Scott JR, Williams A, Fraser H Ultrastructural features of spongiform encephalopathy
Vet Rec. 1991 Oct 5;129(14):320 Synge BA, et al. Spongiform encephalopathy in a Scottish cat.
Vet Rec 1991 Sep 14;129(11):233-6 Wyatt JM, Pearson GR, Naturally occurring scrapie-like s
Vet Rec. 1991 Jun 1;128(22):532. Pearson GR, et al. Feline spongiform encephalopathy.
Vet Rec. 1991 Mar 30;128(13):311. Kock R. Spongiform encephalopathies in ungulates.
Vet Rec. 1991 Feb 2;128(5):115. Gibson PH. Spongiform encephalopathies in ungulates. comment
Vet Rec 1990 Dec 15;127(24):586-8 Leggett MM, Dukes J, Pirie HM A spongiform encephalopathy in a cat.
Done JT. Vet Rec. 1990 Nov 10;127(19):484. Spongiform encephalopathy in pigs.
Vet Rec. 1990 Oct 27;127(17):418-20. Kirkwood JK, et al. Spongiform encephalopathy in an arabian oryx (Oryx leucoryx) and a greater kudu.
Vet Rec. 1990 Sep 29;127(13):338. Dawson M, et al. Primary parenteral transmission of bovine spongiform encephalopathy to the pig.
Vet Rec. 1990 May 19;126(20):513 no authors listed Spongiform encephalopathy in a cat.
Vet Rec 1990 May 12;126(19):489-90 Gibson PH Spongiform encephalopathy in an eland.
Nature. 1990 Mar 15;344(6263):183 Aldhous P. Antelopes die of "mad cow" disease.
Vet Rec 1990 Apr 21;126(16):408-9 Fleetwood AJ, Furley CW Spongiform encephalopathy in an eland.
Vet Pathol. 1988 Sep;25(5):398-9 Jeffrey M, Wells GA Spongiform encephalopathy in a nyala (Tragelaphus angasi) Lasswade Veterinary Laboratory, Midlothian
The BSE Inquiry / Statement No 324
Dr James Kirkwood
(not scheduled to give oral evidence)
Statement to the BSE Inquiry
James K Kirkwood BVSc PhD FIBiol MRCVS
[This witness has not been asked to give oral evidence in Phase 1 of the Inquiry] 1. I became involved in the field of TSEs through my work as Head of the Veterinary Science Group at the Zoological Society of London’s Institute of Zoology. I held this post from November 1984 until June 1996, when I took up my present post at UFAW. During this time, concurrent with the BSE epidemic, cases of scrapie-like spongiform encephalopathies occurred in animals at the Zoological Society of London’s collections at Regent’s Park and Whipsnade and in other zoos. It was appropriate to investigate the epidemiology of these cases in order to try to determine the possible impact on zoo animals and breeding programmes, and to consider how the disease in zoo animals might be controlled.
2. Throughout the period from 1985 to March 1996, I worked at the Institute of Zoology (IoZ). I was Head of the Veterinary Science Group of the IoZ and Senior Veterinary Officer of the Zoological Society of London (ZSL). I was responsible for the provision of the veterinary service for the ZSL collections.
3. During the period from 1985 to March 1996, scrapie-like spongiform encephalopathies were diagnosed in the following animals which died, or were euthanased, at London Zoo and Whipsnade:
Animal Sex Date of Death Age (mos)
Arabian Oryx Oryx leucoryx F 24.3.89 38
Greater kudu Tragelaphus strepsiceros (Linda) F 18.8.89 30
Greater kudu (Karla) F 13.11.90 19
Greater kudu (Kaz) M 6.6.91 37
Greater kudu (Bambi) M 24.10.91 36
Greater kudu (346/90) M 26.2.92 18
Greater kudu (324/90) F 22.11.92 38
Cheetah Acinonyx jubatus (Michelle) F 22.12.93 91
All these cases were described in papers published in the scientific literature (as cited below).
4. All the animals listed above were bred in captivity. The greater kudu were from a highlyinbred group whose founders were Koo (imported from West Africa in 1967), Doo (imported from a Danish Zoo in 1969) and Chester (transferred to London from Chester Zoo in 1982). The family tree of the group is shown in: Kirkwood, J.K., Cunningham, A.A., Wells, G.A.H., Wilesmith, J.W. & Barnett, J.E.F. (1993) Spongiform encephalopathy in a herd of Greater kudu Tragelaphus strepsiceros: epidemiological observations. Veterinary Record 133, 360-364: (J/VR/133/360)
5. The first case diagnosed among the ZSL’s animals was in the greater kudu ‘Linda’, which died in August 1989. Retrospective examination of the brain of the Arabian oryx that had died 5 months earlier, revealed that this animal also had brain lesions characteristic of a scrapielike spongiform encephalopathy. Diagnostic histopathology of these (and of all the other cases that occurred at London and Whipsnade) was undertaken by the Central Veterinary Laboratory. The clinical features, diagnosis and possible aetiology of these first two ZSL cases was discussed in a paper published in 1990 (Kirkwood, J.K., Wells, G.A.H., Wilesmith, J.W., Cunningham, A.A. & Jackson, S.I. (1990) Spongiform encephalopathy in an Arabian oryx Oryx leucoryx and a greater kudu Tragelaphus strepsiceros. Veterinary Record 127, 418-420).(J/VR/127/418). We noted, in this paper, that it seemed probable that these cases had a common aetiology with BSE.
6. A greater kudu ‘Frances’ which had died some 18 months earlier (17.11.87) had shown clinical signs which, in retrospect, could have been due to SE but CNS tissue had not been saved for examination so this could not be checked (Kirkwood, J.K., Cunningham, A.A., Wells, G.A.H., Wilesmith, J.W. & Barnett, J.E.F. (1993) Spongiform encephalopathy in a herd of Greater kudu Tragelaphus strepsiceros: epidemiological observations. Veterinary Record 133, 360-364)(J/VR/133/360).
7. During the following 3 years, SE was diagnosed in 5 further greater kudu in the ZSL collections (see list in point 3 above). The second confirmed case in a greater kudu occurred in the 19-month old calf (Karla) born to the first confirmed case (Linda). This case gave us concern since the calf was born after the July 1988 ban on inclusion of ruminant derived protein in ruminant feeds and it was considered to be extremely unlikely that this animal could have been exposed to contaminated feeds (the kudu diet prior to February 1987 had included a cattle pellet but pelleted diets fed from then on were thought not to contain RDP). We speculated that maternal transmission may have occurred (Kirkwood, J.K., Wells, G.A.H., Cunningham, A.A., Jackson, S.I., Scott, A.C., Dawson, M. & Wilesmith, J.W. (1992). Scrapie-like encephalopathy in greater kudu (Tragelaphus strepsiceros) which had not been fed on ruminant-derived protein. Veterinary Record 130, 365-367:J/VR/130/365).
8. Since the next three animals in which the disease was confirmed (Kaz, Bambi and 346/90) were not thought to have been exposed to contaminated feeds, and were not born to dams who had been clinical cases (Cunningham, A.A., Wells, G.A.H., Scott, A.C., Kirkwood, J.K. & Barnett, J.E.F. (1993) Transmissible spongiform encephalopathy in greater kudu (Tragelaphus strepsiceros). Veterinary Record 132, 68), we considered the possibility that horizontal transmission may have occurred (Kirkwood, J.K., Cunningham, A.A., Wells, G.A.H., Wilesmith, J.W. & Barnett, J.E.F. (1993) Spongiform encephalopathy in a herd of Greater kudu Tragelaphus strepsiceros: epidemiological observations. Veterinary Record 133, 360-364: J/VR/132/68). The occurrence of SE in a greater kudu (324/90), that had been born in another zoo and was not thought to have been exposed to feeds contaminated with RDP, 27 months after being introduced to the group at Regent’s Park, was further cause for concern that transmission may have occurred between animals (Kirkwood, J.K., Cunningham, A.A., Austin, A.R.,Wells, G.A.H & Sainsbury, A.W. (1994) Spongiform encephalopathy in a Greater kudu Tragelaphus strepsiceros introduced into an affected group. Veterinary Record 134, 167-168: J/VR/134/167).
9. At that time, around 1993, the most likely explanation of the pattern of events seemed to be that the disease in kudu was the same as that in cattle: that it had originally entered the group in contaminated feed but that thereafter transmission may have occurred between individuals (Cunningham, A.A., Wells, G.A.H., Scott, A.C., Kirkwood, J.K. & Barnett, J.E.F. (1993) Transmissible spongiform encephalopathy in greater kudu (Tragelaphus strepsiceros).Veterinary Record 132, 68). In view of this and the likelihood that individuals of a wide range of species of zoo animals had been exposed we recommended (Kirkwood, J.K., Cunningham, A.A., Wells, G.A.H., Wilesmith, J.W. & Barnett, J.E.F. (1993) Spongiform encephalopathy in a herd of Greater kudu Tragelaphus strepsiceros: epidemiological observations. Veterinary Record 133, 360-364 ) that all zoo animals that may have been exposed to contaminated feeds should be observed closely and that because of the potentially serious implications for captive breeding programmes, well-described by my colleague Mr Andrew Cunningham (Cunningham, A.A. (1991) Bovine spongiform encephalopathy and British Zoos. Journal of Zoo and Wildlife Medicine 11, 605-634: J/ZWM/11/605), there was a need for caution about exporting such animals. In addition the kudu were kept isolated from other zoo animals. A very cautious approach was taken and the keeper staff used separate tools for cleaning out the kudu dens and paddocks, changed overalls and boots, used latex gloves, and, for a period until it seemed (for reasons mentioned below) less likely that the situation in kudu differed from that in cattle, collected wastes for incineration. Management practices were reviewed again in March 1996 when it was announced that cases of new variant CJD had occurred which might be related to the BSE agent. In order to pre-empt any public concern that might follow this announcement, the walkways past the kudu paddock were closed to the public. No cases have occurred in the kudu group since 1992.
10. The case of SE in a cheetah that occurred during the period, involved a 7 year-old female which had been born and lived all her life at Whipsnade (except for the final stages when she was moved to the Animal Hospital at Regent’s Park for diagnosis and treatment). This animal, which died in December 1993, had been fed on cuts of meat and bone from carcases of cattle unfit for human consumption and it was thought likely that she had been exposed to spinal cord (Kirkwood, J.K., Cunningham, A.A., Flach, E.J., Thornton, S.M. & Wells, G.A.H. (1995) Spongiform encephalopathy in another captive cheetah (Acinonyx jubatus): evidence for variation in susceptibility or incubation periods between species. Journal of Zoo and Wildlife Medicine 26, 577-582: J/ZWM/26/577).
11. During the period we also collated information on cases of SE that occurred in wild animals at or from other zoos in the British Isles. The total number of cases of which I was aware in June 1996, when I presented a review on occurrence of spongiform encephalopathies in zoo animals (at the Royal College of Pathologists’ Symposium on Transmitting prions: BSE, CJD, and other TSEs, The Royal Society, London, 4th July 1996), was 25, involving 10 species. The animals involved were all from the families Bovidae and Felidae, and comprised: 1 Nyala Tragelaphus angasi, 5 Eland Taurotragus oryx, 6 greater kudu Tragelaphus strepsiceros, 1 Gemsbok Oryx gazella, 1 Arabian oryx Oryx leucoryx, 1 Scimitar-horned oryx Oryx dammah, 4 Cheetah Acinonyx jubatus, 3 Puma Felis concolor 2 Ocelot Felis pardalis, and 1 Tiger Panthera tigris. (A spongiform encephalopathy, which was thought probably to have a different aetiology, had also been reported in 3 ostriches Struthio camelus in Germany). This list did not include cases of BSE in domesticated species in zoos (ie BSE in Ankole or other cattle, or SEs, assumed to be scrapie, in mouflon sheep Ovis musimon).
12. Since the time the above statistics were published, a few further cases have occurred in animals at or from zoos in the British Isles. The total number of cases in cheetah that have now been documented has, as far as I am aware, risen to seven (Vitaud, C., Flach, E.J., Thornton, S.M. & Capello, R. (1998) Clinical observations on four cases of feline spongiform encephalopathy in cheetahs (Acinonyx jubatus). Proceedings of the European Association of Zoo and Wildlife Veterinarians, Chester, UK, 21st-24th May 1998. Pp 133-138). There has also been a case in a bison.
13. Epidemiological aspects of the majority of these cases (those diagnosed up to the end of 1993) were considered in paper published in 1994 (Kirkwood, J.K. & Cunningham, A.A. (1994) Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Veterinary Record 135, 296-303:J/VR/135/296.) This paper was based on a paper presented at the Consultation on BSE with the Scientific Veterinary Committee of the Commission of the European Communities held in Brussels, 14-15th September 1993 (Kirkwood, J.K. & Cunningham, A.A. (1993) Spongiform encephalopathy in captive wild animals in Britain: epidemiological observations. In R. Bradley & B Marchant (Eds) Transmissible spongiform encephalopathies. Proceedings of a Consultation on BSE with the Scientific Veterinary Committee of the Commission of the European Communities, 14-15 September 1993, Brussels. European Commission. Pp 29-47:M9 tab 46). It was thought likely that at least some, and probably all, of the cases in zoo animals were caused by the BSE agent. Strong support for this hypothesis came from the findings of Bruce and others (1994) ( Bruce, M.E., Chree, A., McConnell, I., Foster, J., Pearson, G. & Fraser, H. (1994) Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and species barrier. Philosophical Transactions of the Royal Society B 343, 405-411: J/PTRSL/343/405), who demonstrated that the pattern of variation in incubation period and lesion profile in six strains of mice inoculated with brain homogenates from an affected kudu and the nyala, was similar to that seen when this panel of mouse strains was inoculated with brain from cattle with BSE. The affected zoo bovids were all from herds that were exposed to feeds that were likely to have contained contaminated ruminant-derived protein and the zoo felids had been exposed, if only occasionally in some cases, to tissues from cattle unfit for human consumption.
14. Among the affected bovids were others (including scimitar horned oryx and eland) which, like some of the kudu, were born some considerable time after the July 1988 ban on inclusion of RDP in ruminant feeds (Kirkwood, J.K. & Cunningham, A.A. (1994) Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Veterinary Record 135, 296-303:J/VR/135/296). The source of infection to these animals was puzzling. However, as it emerged that many cases of BSE were continuing to occur in domestic cattle born after the July 1988 ban on inclusion of RDP in ruminant feeds, it was clear that the ban had not been immediately effective, and it was therefore possible (or, at least, impossible to rule out) that the late cases in zoo ungulates were also due to exposure to contaminated feeds.
15. We drew attention to the fact that, from a taxonomic perspective, the incidence of cases was strikingly patchy (Kirkwood, J.K. & Cunningham, A.A. (1994) Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Veterinary Record 135, 296-303. Also Kirkwood, J.K., Cunningham, A.A., Flach, E.J., Thornton, S.M. & Wells, G.A.H. (1995) Spongiform encephalopathy in another captive cheetah (Acinonyx jubatus): evidence for variation in susceptibility or incubation periods between species? Journal of Zoo and Wildlife Medicine 26, 577-582) Compared with many other species of exotic ruminants, few kudu were present in the UK but there had been 6 cases of SE among them. The picture seemed similar in the felids. Compared with other species of exotic felids (eg lions in which no cases had occurred), there were relatively small numbers of puma and cheetah in the UK but (at that time) there had been 3 and 4 cases among these respectively. Almost certainly a wider range of species were exposed to contaminated feeds than those in which cases have occurred or been detected. However, we were cautious about drawing firm conclusions about variation in susceptibility between species because (i) incubation periods vary between species and we thought other cases may emerge and (ii) because the variation might be related to differences in intensity of exposure.
TSEs in Exotic Ruminants
TSEs have been detected in exotic ruminants in UK zoos since 1986. These include antelopes (Eland, Gemsbok, Arabian and Scimitar oryx, Nyala and Kudu), Ankole cattle and Bison. With hindsight the 1986 case in a Nyala was diagnosed before the first case of BSE was identified. The TSE cases in exotic ruminants had a younger onset age and a shorter clinical duration compared to that in cattle with BSE. All the cases appear to be linked to the BSE epidemic via the consumption of feed contaminated with the BSE agent. The epidemic has declined as a result of tight controls on feeding mammalian meat and bone meal to susceptible animals, particularly from August 1996.
***> READ THIS VERY, VERY, CAREFULLY, AUGUST 1997 MAD COW FEED BAN WAS A SHAM, AS I HAVE STATED SINCE 1997! 3 FAILSAFES THE FDA ET AL PREACHED AS IF IT WERE THE GOSPEL, IN TERMS OF MAD COW BSE DISEASE IN USA, AND WHY IT IS/WAS/NOT A PROBLEM FOR THE USA, and those are;
BSE TESTING (failed terribly and proven to be a sham)
BSE SURVEILLANCE (failed terribly and proven to be a sham)
BSE 589.2001 FEED REGULATIONS (another colossal failure, and proven to be a sham)
these are facts folks. trump et al just admitted it with the feed ban.
see;
FDA Reports on VFD Compliance
John Maday
August 30, 2019 09:46 AM VFD-Form 007 (640x427)
Before and after the current Veterinary Feed Directive rules took full effect in January, 2017, the FDA focused primarily on education and outreach. ( John Maday ) Before and after the current Veterinary Feed Directive (VFD) rules took full effect in January, 2017, the FDA focused primarily on education and outreach to help feed mills, veterinarians and producers understand and comply with the requirements. Since then, FDA has gradually increased the number of VFD inspections and initiated enforcement actions when necessary. On August 29, FDA released its first report on inspection and compliance activities. The report, titled “Summary Assessment of Veterinary Feed Directive Compliance Activities Conducted in Fiscal Years 2016 – 2018,” is available online.
SUNDAY, SEPTEMBER 1, 2019
***> FDA Reports on VFD Compliance
TUESDAY, APRIL 18, 2017
*** EXTREME USA FDA PART 589 TSE PRION FEED LOOP HOLE STILL EXIST, AND PRICE OF POKER GOES UP ***
THURSDAY, SEPTEMBER 26, 2019
Veterinary Biologics Guideline 3.32E: Guideline for minimising the risk of introducing transmissible spongiform encephalopathy prions and other infectious agents through veterinary biologics
U.S.A. 50 STATE BSE MAD COW CONFERENCE CALL Jan. 9, 2001
Subject: BSE--U.S. 50 STATE CONFERENCE CALL Jan. 9, 2001
Date: Tue, 9 Jan 2001 16:49:00 -0800
From: "Terry S. Singeltary Sr."
Reply-To: Bovine Spongiform Encephalopathy
snip...
[host Richard Barns] and now a question from Terry S. Singeltary of CJD Watch.
[TSS] yes, thank you, U.S. cattle, what kind of guarantee can you give for serum or tissue donor herds?
[no answer, you could hear in the back ground, mumbling and 'we can't. have him ask the question again.]
[host Richard] could you repeat the question?
[TSS] U.S. cattle, what kind of guarantee can you give for serum or tissue donor herds?
[not sure whom ask this] what group are you with?
[TSS] CJD Watch, my Mom died from hvCJD and we are tracking CJD world-wide.
[not sure who is speaking] could you please disconnect Mr. Singeltary
[TSS] you are not going to answer my question?
[not sure whom speaking] NO
snip...see full archive and more of this;
3.2.1.2 Non‐cervid domestic species
The remarkably high rate of natural CWD transmission in the ongoing NA epidemics raises the question of the risk to livestock grazing on CWD‐contaminated shared rangeland and subsequently developing a novel CWD‐related prion disease. This issue has been investigated by transmitting CWD via experimental challenge to cattle, sheep and pigs and to tg mouse lines expressing the relevant species PrP.
For cattle challenged with CWD, PrPSc was detected in approximately 40% of intracerebrally inoculated animals (Hamir et al., 2005, 2006a, 2007). Tg mice expressing bovine PrP have also been challenged with CWD and while published studies have negative outcomes (Tamguney et al., 2009b), unpublished data provided for the purposes of this Opinion indicate that some transmission of individual isolates to bovinised mice is possible (Table 1).
In small ruminant recipients, a low rate of transmission was reported between 35 and 72 months post‐infection (mpi) in ARQ/ARQ and ARQ/VRQ sheep intracerebrally challenged with mule deer CWD (Hamir et al., 2006b), while two out of two ARQ/ARQ sheep intracerebrally inoculated with elk CWD developed clinical disease after 28 mpi (Madsen‐Bouterse et al., 2016). However, tg mice expressing ARQ sheep PrP were resistant (Tamguney et al., 2006) and tg mice expressing the VRQ PrP allele were poorly susceptible to clinical disease (Beringue et al., 2012; Madsen‐Bouterse et al., 2016). In contrast, tg mice expressing VRQ sheep PrP challenged with CWD have resulted in highly efficient, life‐long asymptomatic replication of these prions in the spleen tissue (Beringue et al., 2012).
A recent study investigated the potential for swine to serve as hosts of the CWD agent(s) by intracerebral or oral challenge of crossbred piglets (Moore et al., 2016b, 2017). Pigs sacrificed at 6 mpi, approximately the age at which pigs reach market weight, were clinically healthy and negative by diagnostic tests, although low‐level CWD agent replication could be detected in the CNS by bioassay in tg cervinised mice. Among pigs that were incubated for up to 73 mpi, some gave diagnostic evidence of CWD replication in the brain between 42 and 72 mpi. Importantly, this was observed also in one orally challenged pig at 64 mpi and the presence of low‐level CWD replication was confirmed by mouse bioassay. The authors of this study argued that pigs can support low‐level amplification of CWD prions, although the species barrier to CWD infection is relatively high and that the detection of infectivity in orally inoculated pigs with a mouse bioassay raises the possibility that naturally exposed pigs could act as a reservoir of CWD infectivity.
MONDAY, MARCH 08, 2021
https://www.nature.com/articles/srep11573
PRION 2016 TOKYO
Saturday, April 23, 2016
SCRAPIE WS-01: Prion diseases in animals and zoonotic potential 2016
Prion. 10:S15-S21. 2016 ISSN: 1933-6896 printl 1933-690X online
Taylor & Francis
Prion 2016 Animal Prion Disease Workshop Abstracts
WS-01: Prion diseases in animals and zoonotic potential
Transmission of the different scrapie isolates in these mice leads to the emergence of prion strain phenotypes that showed similar characteristics to those displayed by MM1 or VV2 sCJD prion.
These results demonstrate that scrapie prions have a zoonotic potential and raise new questions about the possible link between animal and human prions.
http://www.tandfonline.com/doi/abs/10.1080/19336896.2016.1163048?journalCode=kprn20
Title: Transmission of scrapie prions to primate after an extended silent incubation period)
*** In complement to the recent demonstration that humanized mice are susceptible to scrapie, we report here the first observation of direct transmission of a natural classical scrapie isolate to a macaque after a 10-year incubation period. Neuropathologic examination revealed all of the features of a prion disease: spongiform change, neuronal loss, and accumulation of PrPres throughout the CNS.
*** This observation strengthens the questioning of the harmlessness of scrapie to humans, at a time when protective measures for human and animal health are being dismantled and reduced as c-BSE is considered controlled and being eradicated.
*** Our results underscore the importance of precautionary and protective measures and the necessity for long-term experimental transmission studies to assess the zoonotic potential of other animal prion strains.
http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=313160
Control of Chronic Wasting Disease OMB Control Number: 0579-0189 APHIS-2021-0004 Singeltary Submission
***> Chronic Wasting Disease CWD TSE Prion Zoonosis Zoonotic Update <***
Cervid to human prion transmission
Kong, Qingzhong
Case Western Reserve University, Cleveland, OH, United States
Prion disease is transmissible and invariably fatal. Chronic wasting disease (CWD) is the prion disease affecting deer, elk and moose, and it is a widespread and expanding epidemic affecting 22 US States and 2 Canadian provinces so far. CWD poses the most serious zoonotic prion transmission risks in North America because of huge venison consumption (>6 million deer/elk hunted and consumed annually in the USA alone), significant prion infectivity in muscles and other tissues/fluids from CWD-affected cervids, and usually high levels of individual exposure to CWD resulting from consumption of the affected animal among often just family and friends. However, we still do not know whether CWD prions can infect humans in the brain or peripheral tissues or whether clinical/asymptomatic CWD zoonosis has already occurred, and we have no essays to reliably detect CWD infection in humans.
We hypothesize that:
(1) The classic CWD prion strain can infect humans at low levels in the brain and peripheral lymphoid tissues;
(2) The cervid-to-human transmission barrier is dependent on the cervid prion strain and influenced by the host (human) prion protein (PrP) primary sequence;
(3) Reliable essays can be established to detect CWD infection in humans; and
(4) CWD transmission to humans has already occurred. We will test these hypotheses in 4 Aims using transgenic (Tg) mouse models and complementary in vitro approaches.
Aim 1 will prove that the classical CWD strain may infect humans in brain or peripheral lymphoid tissues at low levels by conducting systemic bioassays in a set of humanized Tg mouse lines expressing common human PrP variants using a number of CWD isolates at varying doses and routes. Experimental human CWD samples will also be generated for Aim 3.
Aim 2 will test the hypothesis that the cervid-to-human prion transmission barrier is dependent on prion strain and influenced by the host (human) PrP sequence by examining and comparing the transmission efficiency and phenotypes of several atypical/unusual CWD isolates/strains as well as a few prion strains from other species that have adapted to cervid PrP sequence, utilizing the same panel of humanized Tg mouse lines as in Aim 1.
Aim 3 will establish reliable essays for detection and surveillance of CWD infection in humans by examining in details the clinical, pathological, biochemical and in vitro seeding properties of existing and future experimental human CWD samples generated from Aims 1-2 and compare them with those of common sporadic human Creutzfeldt-Jakob disease (sCJD) prions.
Aim 4 will attempt to detect clinical CWD-affected human cases by examining a significant number of brain samples from prion-affected human subjects in the USA and Canada who have consumed venison from CWD-endemic areas utilizing the criteria and essays established in Aim 3. The findings from this proposal will greatly advance our understandings on the potential and characteristics of cervid prion transmission in humans, establish reliable essays for CWD zoonosis and potentially discover the first case(s) of CWD infection in humans.
Public Health Relevance
There are significant and increasing human exposure to cervid prions because chronic wasting disease (CWD, a widespread and highly infectious prion disease among deer and elk in North America) continues spreading and consumption of venison remains popular, but our understanding on cervid-to-human prion transmission is still very limited, raising public health concerns. This proposal aims to define the zoonotic risks of cervid prions and set up and apply essays to detect CWD zoonosis using mouse models and in vitro methods. The findings will greatly expand our knowledge on the potentials and characteristics of cervid prion transmission in humans, establish reliable essays for such infections and may discover the first case(s) of CWD infection in humans.
International Conference on Emerging Diseases, Outbreaks & Case Studies & 16th Annual Meeting on Influenza March 28-29, 2018 | Orlando, USA
Qingzhong Kong
Case Western Reserve University School of Medicine, USA
Zoonotic potential of chronic wasting disease prions from cervids
Chronic wasting disease (CWD) is the prion disease in cervids (mule deer, white-tailed deer, American elk, moose, and reindeer). It has become an epidemic in North America, and it has been detected in the Europe (Norway) since 2016. The widespread CWD and popular hunting and consumption of cervid meat and other products raise serious public health concerns, but questions remain on human susceptibility to CWD prions, especially on the potential difference in zoonotic potential among the various CWD prion strains. We have been working to address this critical question for well over a decade. We used CWD samples from various cervid species to inoculate transgenic mice expressing human or elk prion protein (PrP). We found infectious prions in the spleen or brain in a small fraction of CWD-inoculated transgenic mice expressing human PrP, indicating that humans are not completely resistant to CWD prions; this finding has significant ramifications on the public health impact of CWD prions. The influence of cervid PrP polymorphisms, the prion strain dependence of CWD-to-human transmission barrier, and the characterization of experimental human CWD prions will be discussed.
Speaker Biography Qingzhong Kong has completed his PhD from the University of Massachusetts at Amherst and Post-doctoral studies at Yale University. He is currently an Associate Professor of Pathology, Neurology and Regenerative Medicine. He has published over 50 original research papers in reputable journals (including Science Translational Medicine, JCI, PNAS and Cell Reports) and has been serving as an Editorial Board Member on seven scientific journals. He has multiple research interests, including public health risks of animal prions (CWD of cervids and atypical BSE of cattle), animal modeling of human prion diseases, mechanisms of prion replication and pathogenesis, etiology of sporadic Creutzfeldt-Jacob disease (CJD) in humans, normal cellular PrP in the biology and pathology of multiple brain and peripheral diseases, proteins responsible for the α-cleavage of cellular PrP, as well as gene therapy and DNA vaccination.
Prion Conference 2018 Abstracts
BSE aka MAD COW DISEASE, was first discovered in 1984, and it took until 1995 to finally admit that BSE was causing nvCJD, the rest there is history, but that science is still evolving i.e. science now shows that indeed atypical L-type BSE, atypical Nor-98 Scrapie, and typical Scrapie are all zoonosis, zoonotic for humans, there from.
HOW long are we going to wait for Chronic Wasting Disease, CWD TSE Prion of Cervid, and zoonosis, zoonotic tranmission to humans there from?
Studies have shown since 1994 that humans are susceptible to CWD TSE Prion, so, what's the hold up with making CWD a zoonotic zoonosis disease, the iatrogenic transmissions there from is not waiting for someone to make a decision.
Prion Conference 2018 Abstracts
P190 Human prion disease mortality rates by occurrence of chronic wasting disease in freeranging cervids, United States
Abrams JY (1), Maddox RA (1), Schonberger LB (1), Person MK (1), Appleby BS (2), Belay ED (1)
(1) Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Atlanta, GA, USA (2) Case Western Reserve University, National Prion Disease Pathology Surveillance Center (NPDPSC), Cleveland, OH, USA.
Background
Chronic wasting disease (CWD) is a prion disease of deer and elk that has been identified in freeranging cervids in 23 US states. While there is currently no epidemiological evidence for zoonotic transmission through the consumption of contaminated venison, studies suggest the CWD agent can cross the species barrier in experimental models designed to closely mimic humans. We compared rates of human prion disease in states with and without CWD to examine the possibility of undetermined zoonotic transmission.
Methods
Death records from the National Center for Health Statistics, case records from the National Prion Disease Pathology Surveillance Center, and additional state case reports were combined to create a database of human prion disease cases from 2003-2015. Identification of CWD in each state was determined through reports of positive CWD tests by state wildlife agencies. Age- and race-adjusted mortality rates for human prion disease, excluding cases with known etiology, were determined for four categories of states based on CWD occurrence: highly endemic (>16 counties with CWD identified in free-ranging cervids); moderately endemic (3-10 counties with CWD); low endemic (1-2 counties with CWD); and no CWD states. States were counted as having no CWD until the year CWD was first identified. Analyses stratified by age, sex, and time period were also conducted to focus on subgroups for which zoonotic transmission would be more likely to be detected: cases <55 years old, male sex, and the latter half of the study (2010-2015).
Results
Highly endemic states had a higher rate of prion disease mortality compared to non-CWD states (rate ratio [RR]: 1.12, 95% confidence interval [CI] = 1.01 - 1.23), as did low endemic states (RR: 1.15, 95% CI = 1.04 - 1.27). Moderately endemic states did not have an elevated mortality rate (RR: 1.05, 95% CI = 0.93 - 1.17). In age-stratified analyses, prion disease mortality rates among the <55 year old population were elevated for moderately endemic states (RR: 1.57, 95% CI = 1.10 – 2.24) while mortality rates were elevated among those ≥55 for highly endemic states (RR: 1.13, 95% CI = 1.02 - 1.26) and low endemic states (RR: 1.16, 95% CI = 1.04 - 1.29). In other stratified analyses, prion disease mortality rates for males were only elevated for low endemic states (RR: 1.27, 95% CI = 1.10 - 1.48), and none of the categories of CWD-endemic states had elevated mortality rates for the latter time period (2010-2015).
Conclusions
While higher prion disease mortality rates in certain categories of states with CWD in free-ranging cervids were noted, additional stratified analyses did not reveal markedly elevated rates for potentially sensitive subgroups that would be suggestive of zoonotic transmission. Unknown confounding factors or other biases may explain state-by-state differences in prion disease mortality.
=====
P172 Peripheral Neuropathy in Patients with Prion Disease
Wang H(1), Cohen M(1), Appleby BS(1,2)
(1) University Hospitals Cleveland Medical Center, Cleveland, Ohio (2) National Prion Disease Pathology Surveillance Center, Cleveland, Ohio.
Prion disease is a fatal progressive neurodegenerative disease due to deposition of an abnormal protease-resistant isoform of prion protein. Typical symptoms include rapidly progressive dementia, myoclonus, visual disturbance and hallucinations. Interestingly, in patients with prion disease, the abnormal protein canould also be found in the peripheral nervous system. Case reports of prion deposition in peripheral nerves have been reported. Peripheral nerve involvement is thought to be uncommon; however, little is known about the exact prevalence and features of peripheral neuropathy in patients with prion disease.
We reviewed autopsy-proven prion cases from the National Prion Disease Pathology Surveillance Center that were diagnosed between September 2016 to March 2017. We collected information regarding prion protein diagnosis, demographics, comorbidities, clinical symptoms, physical exam, neuropathology, molecular subtype, genetics lab, brain MRI, image and EMG reports. Our study included 104 patients. Thirteen (12.5%) patients had either subjective symptoms or objective signs of peripheral neuropathy. Among these 13 patients, 3 had other known potential etiologies of peripheral neuropathy such as vitamin B12 deficiency or prior chemotherapy. Among 10 patients that had no other clear etiology, 3 (30%) had familial CJD. The most common sCJD subtype was MV1-2 (30%), followed by MM1-2 (20%). The Majority of cases wasere male (60%). Half of them had exposure to wild game. The most common subjective symptoms were tingling and/or numbness of distal extremities. The most common objective finding was diminished vibratory sensation in the feet. Half of them had an EMG with the findings ranging from fasciculations to axonal polyneuropathy or demyelinating polyneuropathy.
Our study provides an overview of the pattern of peripheral neuropathy in patients with prion disease. Among patients with peripheral neuropathy symptoms or signs, majority has polyneuropathy. It is important to document the baseline frequency of peripheral neuropathy in prion diseases as these symptoms may become important when conducting surveillance for potential novel zoonotic prion diseases.
=====
P177 PrP plaques in methionine homozygous Creutzfeldt-Jakob disease patients as a potential marker of iatrogenic transmission
Abrams JY (1), Schonberger LB (1), Cali I (2), Cohen Y (2), Blevins JE (2), Maddox RA (1), Belay ED (1), Appleby BS (2), Cohen ML (2)
(1) Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Atlanta, GA, USA (2) Case Western Reserve University, National Prion Disease Pathology Surveillance Center (NPDPSC), Cleveland, OH, USA.
Background
Sporadic Creutzfeldt-Jakob disease (CJD) is widely believed to originate from de novo spontaneous conversion of normal prion protein (PrP) to its pathogenic form, but concern remains that some reported sporadic CJD cases may actually be caused by disease transmison via iatrogenic processes. For cases with methionine homozygosity (CJD-MM) at codon 129 of the PRNP gene, recent research has pointsied to plaque-like PrP deposition as a potential marker of iatrogenic transmission for a subset of cases. This phenotype is theorized to originate from specific iatrogenic source CJD types that comprise roughly a quarter of known CJD cases.
Methods
We reviewed scientific literature for studies which described PrP plaques among CJD patients with known epidemiological links to iatrogenic transmission (receipt of cadaveric human grown hormone or dura mater), as well as in cases of reported sporadic CJD. The presence and description of plaques, along with CJD classification type and other contextual factors, were used to summarize the current evidence regarding plaques as a potential marker of iatrogenic transmission. In addition, 523 cases of reported sporadic CJD cases in the US from January 2013 through September 2017 were assessed for presence of PrP plaques.
Results
We identified four studies describing 52 total cases of CJD-MM among either dura mater recipients or growth hormone recipients, of which 30 were identified as having PrP plaques. While sporadic cases were not generally described as having plaques, we did identify case reports which described plaques among sporadic MM2 cases as well as case reports of plaques exclusively in white matter among sporadic MM1 cases. Among the 523 reported sporadic CJD cases, 0 of 366 MM1 cases had plaques, 2 of 48 MM2 cases had kuru plaques, and 4 of 109 MM1+2 cases had either kuru plaques or both kuru and florid plaques. Medical chart review of the six reported sporadic CJD cases with plaques did not reveal clinical histories suggestive of potential iatrogenic transmission.
Conclusions
PrP plaques occur much more frequently for iatrogenic CJD-MM cases compared to sporadic CJDMM cases. Plaques may indicate iatrogenic transmission for CJD-MM cases without a type 2 Western blot fragment. The study results suggest the absence of significant misclassifications of iatrogenic CJD as sporadic. To our knowledge, this study is the first to describe grey matter kuru plaques in apparently sporadic CJD-MM patients with a type 2 Western blot fragment.
=====
P180 Clinico-pathological analysis of human prion diseases in a brain bank series
Ximelis T (1), Aldecoa I (1,2), Molina-Porcel L (1,3), Grau-Rivera O (4), Ferrer I (5), Nos C (6), Gelpi E (1,7), Sánchez-Valle R (1,4)
(1) Neurological Tissue Bank of the Biobanc-Hospital ClÃnic-IDIBAPS, Barcelona, Spain (2) Pathological Service of Hospital ClÃnic de Barcelona, Barcelona, Spain (3) EAIA Trastorns Cognitius, Centre Emili Mira, Parc de Salut Mar, Barcelona, Spain (4) Department of Neurology of Hospital ClÃnic de Barcelona, Barcelona, Spain (5) Institute of Neuropathology, Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Barcelona (6) General subdirectorate of Surveillance and Response to Emergencies in Public Health, Department of Public Health in Catalonia, Barcelona, Spain (7) Institute of Neurology, Medical University of Vienna, Vienna, Austria.
Background and objective:
The Neurological Tissue Bank (NTB) of the Hospital Clínic-Institut d‘Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain is the reference center in Catalonia for the neuropathological study of prion diseases in the region since 2001. The aim of this study is to analyse the characteristics of the confirmed prion diseases registered at the NTB during the last 15 years.
Methods:
We reviewed retrospectively all neuropathologically confirmed cases registered during the period January 2001 to December 2016.
Results:
176 cases (54,3% female, mean age: 67,5 years and age range: 25-86 years) of neuropathological confirmed prion diseases have been studied at the NTB. 152 cases corresponded to sporadic Creutzfeldt-Jakob disease (sCJD), 10 to genetic CJD, 10 to Fatal Familial Insomnia, 2 to GerstmannSträussler-Scheinker disease, and 2 cases to variably protease-sensitive prionopathy (VPSPr). Within sCJD subtypes the MM1 subtype was the most frequent, followed by the VV2 histotype.
Clinical and neuropathological diagnoses agreed in 166 cases (94%). The clinical diagnosis was not accurate in 10 patients with definite prion disease: 1 had a clinical diagnosis of Fronto-temporal dementia (FTD), 1 Niemann-Pick‘s disease, 1 Lewy Body‘s Disease, 2 Alzheimer‘s disease, 1 Cortico-basal syndrome and 2 undetermined dementia. Among patients with VPSPr, 1 had a clinical diagnosis of Amyotrophic lateral sclerosis (ALS) and the other one with FTD.
Concomitant pathologies are frequent in older age groups, mainly AD neuropathological changes were observed in these subjects.
Discussion:
A wide spectrum of human prion diseases have been identified in the NTB being the relative frequencies and main characteristics like other published series. There is a high rate of agreement between clinical and neuropathological diagnoses with prion diseases. These findings show the importance that public health has given to prion diseases during the past 15 years. Continuous surveillance of human prion disease allows identification of new emerging phenotypes. Brain tissue samples from these donors are available to the scientific community. For more information please visit:
=====
P192 Prion amplification techniques for the rapid evaluation of surface decontamination procedures
Bruyere-Ostells L (1), Mayran C (1), Belondrade M (1), Boublik Y (2), Haïk S (3), Fournier-Wirth C (1), Nicot S (1), Bougard D (1)
(1) Pathogenesis and control of chronic infections, Etablissement Français du Sang, Inserm, Université de Montpellier, Montpellier, France. (2) Centre de Recherche en Biologie cellulaire de Montpellier, CNRS, Université de Montpellier, Montpellier, France. (3) Inserm U 1127, CNRS UMR 7225, Sorbonne Universités, UPMC Université Paris 06 UMR S 1127, Institut du Cerveau et de la Moelle épinière, ICM, Paris, France.
Aims:
Transmissible Spongiform Encephalopathies (TSE) or prion diseases are a group of incurable and always fatal neurodegenerative disorders including Creutzfeldt-Jakob diseases (CJD) in humans. These pathologies include sporadic (sCJD), genetic and acquired (variant CJD) forms. By the past, sCJD and vCJD were transmitted by different prion contaminated biological materials to patients resulting in more than 400 iatrogenic cases (iCJD). The atypical nature and the biochemical properties of the infectious agent, formed by abnormal prion protein or PrPTSE, make it particularly resistant to conventional decontamination procedures. In addition, PrPTSE is widely distributed throughout the organism before clinical onset in vCJD and can also be detected in some peripheral tissues in sporadic CJD. Risk of iatrogenic transmission of CJD by contaminated medical device remains thus a concern for healthcare facilities. Bioassay is the gold standard method to evaluate the efficacy of prion decontamination procedures but is time-consuming and expensive. Here, we propose to compare in vitro prion amplification techniques: Protein Misfolding Cyclic Amplification (PMCA) and Real-Time Quaking Induced Conversion (RT-QuIC) for the detection of residual prions on surface after decontamination.
Methods:
Stainless steel wires, by mimicking the surface of surgical instruments, were proposed as a carrier model of prions for inactivation studies. To determine the sensitivity of the two amplification techniques on wires (Surf-PMCA and Surf-QuIC), steel wires were therefore contaminated with serial dilutions of brain homogenates (BH) from a 263k infected hamster and from a patient with sCJD (MM1 subtype). We then compared the different standard decontamination procedures including partially and fully efficient treatments by detecting the residual seeding activity on 263K and sCJD contaminated wires. We completed our study by the evaluation of marketed reagents endorsed for prion decontamination.
Results:
The two amplification techniques can detect minute quantities of PrPTSE adsorbed onto a single wire. 8/8 wires contaminated with a 10-6 dilution of 263k BH and 1/6 with the 10-8 dilution are positive with Surf-PMCA. Similar performances were obtained with Surf-QuIC on 263K: 10/16 wires contaminated with 10-6 dilution and 1/8 wires contaminated with 10-8 dilution are positive. Regarding the human sCJD-MM1 prion, Surf-QuIC allows us to detect 16/16 wires contaminated with 10-6 dilutions and 14/16 with 10-7 . Results obtained after decontamination treatments are very similar between 263K and sCJD prions. Efficiency of marketed treatments to remove prions is lower than expected.
Conclusions:
Surf-PMCA and Surf-QuIC are very sensitive methods for the detection of prions on wires and could be applied to prion decontamination studies for rapid evaluation of new treatments. Sodium hypochlorite is the only product to efficiently remove seeding activity of both 263K and sCJD prions.
=====
WA2 Oral transmission of CWD into Cynomolgus macaques: signs of atypical disease, prion conversion and infectivity in macaques and bio-assayed transgenic mice
Schatzl HM (1, 2), Hannaoui S (1, 2), Cheng Y-C (1, 2), Gilch S (1, 2), Beekes M (3), SchulzSchaeffer W (4), Stahl-Hennig C (5) and Czub S (2, 6)
(1) University of Calgary, Calgary Prion Research Unit, Calgary, Canada (2) University of Calgary, Faculty of Veterinary Medicine, Calgary, Canada, (3) Robert Koch Institute, Berlin, Germany, (4) University of Homburg/Saar, Homburg, Germany, (5) German Primate Center, Goettingen, Germany, (6) Canadian Food Inspection Agency (CFIA), Lethbridge, Canada.
To date, BSE is the only example of interspecies transmission of an animal prion disease into humans. The potential zoonotic transmission of CWD is an alarming issue and was addressed by many groups using a variety of in vitro and in vivo experimental systems. Evidence from these studies indicated a substantial, if not absolute, species barrier, aligning with the absence of epidemiological evidence suggesting transmission into humans. Studies in non-human primates were not conclusive so far, with oral transmission into new-world monkeys and no transmission into old-world monkeys. Our consortium has challenged 18 Cynomolgus macaques with characterized CWD material, focusing on oral transmission with muscle tissue. Some macaques have orally received a total of 5 kg of muscle material over a period of 2 years. After 5-7 years of incubation time some animals showed clinical symptoms indicative of prion disease, and prion neuropathology and PrPSc deposition were found in spinal cord and brain of euthanized animals. PrPSc in immunoblot was weakly detected in some spinal cord materials and various tissues tested positive in RT-QuIC, including lymph node and spleen homogenates. To prove prion infectivity in the macaque tissues, we have intracerebrally inoculated 2 lines of transgenic mice, expressing either elk or human PrP. At least 3 TgElk mice, receiving tissues from 2 different macaques, showed clinical signs of a progressive prion disease and brains were positive in immunoblot and RT-QuIC. Tissues (brain, spinal cord and spleen) from these and preclinical mice are currently tested using various read-outs and by second passage in mice. Transgenic mice expressing human PrP were so far negative for clear clinical prion disease (some mice >300 days p.i.). In parallel, the same macaque materials are inoculated into bank voles. Taken together, there is strong evidence of transmissibility of CWD orally into macaques and from macaque tissues into transgenic mouse models, although with an incomplete attack rate. The clinical and pathological presentation in macaques was mostly atypical, with a strong emphasis on spinal cord pathology. Our ongoing studies will show whether the transmission of CWD into macaques and passage in transgenic mice represents a form of non-adaptive prion amplification, and whether macaque-adapted prions have the potential to infect mice expressing human PrP. The notion that CWD can be transmitted orally into both new-world and old-world non-human primates asks for a careful reevaluation of the zoonotic risk of CWD.
See also poster P103
***> The notion that CWD can be transmitted orally into both new-world and old-world non-human primates asks for a careful reevaluation of the zoonotic risk of CWD.
=====
WA16 Monitoring Potential CWD Transmission to Humans
Belay ED
Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Atlanta, GA, USA.
The spread of chronic wasting disease (CWD) in animals has raised concerns about increasing human exposure to the CWD agent via hunting and venison consumption, potentially facilitating CWD transmission to humans. Several studies have explored this possibility, including limited epidemiologic studies, in vitro experiments, and laboratory studies using various types of animal models. Most human exposures to the CWD agent in the United States would be expected to occur in association with deer and elk hunting in CWD-endemic areas. The Centers for Disease Control and Prevention (CDC) collaborated with state health departments in Colorado, Wisconsin, and Wyoming to identify persons at risk of CWD exposure and to monitor their vital status over time. Databases were established of persons who hunted in Colorado and Wyoming and those who reported consumption of venison from deer that later tested positive in Wisconsin. Information from the databases is periodically cross-checked with mortality data to determine the vital status and causes of death for deceased persons. Long-term follow-up of these hunters is needed to assess their risk of development of a prion disease linked to CWD exposure.
=====
P166 Characterization of CJD strain profiles in venison consumers and non-consumers from Alberta and Saskatchewan
Stephanie Booth (1,2), Lise Lamoureux (1), Debra Sorensen (1), Jennifer L. Myskiw (1,2), Megan Klassen (1,2), Michael Coulthart (3), Valerie Sim (4)
(1) Zoonotic Diseases and Special Pathogens, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg (2) Department of Medical Microbiology and Infectious Diseases, University of Manitoba, Winnipeg (3) Canadian CJD Surveillance System, Public Health Agency of Canada, Ottawa (4) Division of Neurology, Department of Medicine Centre for Prions and Protein Folding Diseases, University of Alberta, Edmonton.
Chronic wasting disease (CWD) is spreading rapidly through wild cervid populations in the Canadian provinces of Alberta and Saskatchewan. While this has implications for tourism and hunting, there is also concern over possible zoonotic transmission to humans who eat venison from infected deer. Whilst there is no evidence of any human cases of CWD to date, the Canadian CJD Surveillance System (CJDSS) in Canada is staying vigilant. When variant CJD occurred following exposure to BSE, the unique biochemical fingerprint of the pathologic PrP enabled a causal link to be confirmed. However, we cannot be sure what phenotype human CWD prions would present with, or indeed, whether this would be distinct from that see in sporadic CJD. Therefore we are undertaking a systematic analysis of the molecular diversity of CJD cases of individuals who resided in Alberta and Saskatchewan at their time of death comparing venison consumers and non-consumers, using a variety of clinical, imaging, pathological and biochemical markers. Our initial objective is to develop novel biochemical methodologies that will extend the baseline glycoform and genetic polymorphism typing that is already completed by the CJDSS. Firstly, we are reviewing MRI, EEG and pathology information from over 40 cases of CJD to select clinically affected areas for further investigation. Biochemical analysis will include assessment of the levels of protease sensitive and resistant prion protein, glycoform typing using 2D gel electrophoresis, testing seeding capabilities and kinetics of aggregation by quaking-induced conversion, and determining prion oligomer size distributions with asymmetric flow field fractionation with in-line light scattering. Progress and preliminary data will be presented. Ultimately, we intend to further define the relationship between PrP structure and disease phenotype and establish a baseline for the identification of future atypical CJD cases that may arise as a result of exposure to CWD.
=====
Source Prion Conference 2018 Abstracts
Volume 24, Number 8—August 2018 Research Susceptibility of Human Prion Protein to Conversion by Chronic Wasting Disease Prions
Marcelo A. BarriaComments to Author , Adriana Libori, Gordon Mitchell, and Mark W. Head Author affiliations: National CJD Research and Surveillance Unit, University of Edinburgh, Edinburgh, Scotland, UK (M.A. Barria, A. Libori, M.W. Head); National and OIE Reference Laboratory for Scrapie and CWD, Canadian Food Inspection Agency, Ottawa, Ontario, Canada (G. Mitchell)
Abstract Chronic wasting disease (CWD) is a contagious and fatal neurodegenerative disease and a serious animal health issue for deer and elk in North America. The identification of the first cases of CWD among free-ranging reindeer and moose in Europe brings back into focus the unresolved issue of whether CWD can be zoonotic like bovine spongiform encephalopathy. We used a cell-free seeded protein misfolding assay to determine whether CWD prions from elk, white-tailed deer, and reindeer in North America can convert the human prion protein to the disease-associated form. We found that prions can convert, but the efficiency of conversion is affected by polymorphic variation in the cervid and human prion protein genes. In view of the similarity of reindeer, elk, and white-tailed deer in North America to reindeer, red deer, and roe deer, respectively, in Europe, a more comprehensive and thorough assessment of the zoonotic potential of CWD might be warranted.
snip...
Discussion Characterization of the transmission properties of CWD and evaluation of their zoonotic potential are important for public health purposes. Given that CWD affects several members of the family Cervidae, it seems reasonable to consider whether the zoonotic potential of CWD prions could be affected by factors such as CWD strain, cervid species, geographic location, and Prnp–PRNP polymorphic variation. We have previously used an in vitro conversion assay (PMCA) to investigate the susceptibility of the human PrP to conversion to its disease-associated form by several animal prion diseases, including CWD (15,16,22). The sensitivity of our molecular model for the detection of zoonotic conversion depends on the combination of 1) the action of proteinase K to degrade the abundant human PrPC that constitutes the substrate while only N terminally truncating any human PrPres produced and 2) the presence of the 3F4 epitope on human but not cervid PrP. In effect, this degree of sensitivity means that any human PrPres formed during the PMCA reaction can be detected down to the limit of Western blot sensitivity. In contrast, if other antibodies that detect both cervid and human PrP are used, such as 6H4, then newly formed human PrPres must be detected as a measurable increase in PrPres over the amount remaining in the reaction product from the cervid seed. Although best known for the efficient amplification of prions in research and diagnostic contexts, the variation of the PMCA method employed in our study is optimized for the definitive detection of zoonotic reaction products of inherently inefficient conversion reactions conducted across species barriers. By using this system, we previously made and reported the novel observation that elk CWD prions could convert human PrPC from human brain and could also convert recombinant human PrPC expressed in transgenic mice and eukaryotic cell cultures (15).
A previous publication suggested that mule deer PrPSc was unable to convert humanized transgenic substrate in PMCA assays (23) and required a further step of in vitro conditioning in deer substrate PMCA before it was able to cross the deer–human molecular barrier (24). However, prions from other species, such as elk (15) and reindeer affected by CWD, appear to be compatible with the human protein in a single round of amplification (as shown in our study). These observations suggest that different deer species affected by CWD could present differing degrees of the olecular compatibility with the normal form of human PrP.
The contribution of the polymorphism at codon 129 of the human PrP gene has been extensively studied and is recognized as a risk factor for Creutzfeldt-Jakob disease (4). In cervids, the equivalent codon corresponds to the position 132 encoding methionine or leucine. This polymorphism in the elk gene has been shown to play an important role in CWD susceptibility (25,26). We have investigated the effect of this cervid Prnp polymorphism on the conversion of the humanized transgenic substrate according to the variation in the equivalent PRNP codon 129 polymorphism. Interestingly, only the homologs methionine homozygous seed–substrate reactions could readily convert the human PrP, whereas the heterozygous elk PrPSc was unable to do so, even though comparable amounts of PrPres were used to seed the reaction. In addition, we observed only low levels of human PrPres formation in the reactions seeded with the homozygous methionine (132 MM) and the heterozygous (132 ML) seeds incubated with the other 2 human polymorphic substrates (129 MV and 129 VV). The presence of the amino acid leucine at position 132 of the elk Prnp gene has been attributed to a lower degree of prion conversion compared with methionine on the basis of experiments in mice made transgenic for these polymorphic variants (26). Considering the differences observed for the amplification of the homozygous human methionine substrate by the 2 polymorphic elk seeds (MM and ML), reappraisal of the susceptibility of human PrPC by the full range of cervid polymorphic variants affected by CWD would be warranted.
In light of the recent identification of the first cases of CWD in Europe in a free-ranging reindeer (R. tarandus) in Norway (2), we also decided to evaluate the in vitro conversion potential of CWD in 2 experimentally infected reindeer (18). Formation of human PrPres was readily detectable after a single round of PMCA, and in all 3 humanized polymorphic substrates (MM, MV, and VV). This finding suggests that CWD prions from reindeer could be more compatible with human PrPC generally and might therefore present a greater risk for zoonosis than, for example, CWD prions from white-tailed deer. A more comprehensive comparison of CWD in the affected species, coupled with the polymorphic variations in the human and deer PRNP–Prnp genes, in vivo and in vitro, will be required before firm conclusions can be drawn. Analysis of the Prnp sequence of the CWD reindeer in Norway was reported to be identical to the specimens used in our study (2). This finding raises the possibility of a direct comparison of zoonotic potential between CWD acquired in the wild and that produced in a controlled laboratory setting. (Table).
The prion hypothesis proposes that direct molecular interaction between PrPSc and PrPC is necessary for conversion and prion replication. Accordingly, polymorphic variants of the PrP of host and agent might play a role in determining compatibility and potential zoonotic risk. In this study, we have examined the capacity of the human PrPC to support in vitro conversion by elk, white-tailed deer, and reindeer CWD PrPSc. Our data confirm that elk CWD prions can convert the human PrPC, at least in vitro, and show that the homologous PRNP polymorphisms at codon 129 and 132 in humans and cervids affect conversion efficiency. Other species affected by CWD, particularly caribou or reindeer, also seem able to convert the human PrP. It will be important to determine whether other polymorphic variants found in other CWD-affected Cervidae or perhaps other factors (17) exert similar effects on the ability to convert human PrP and thus affect their zoonotic potential.
Dr. Barria is a research scientist working at the National CJD Research and Surveillance Unit, University of Edinburgh. His research has focused on understanding the molecular basis of a group of fatal neurologic disorders called prion diseases.
Acknowledgments We thank Aru Balachandran for originally providing cervid brain tissues, Abigail Diack and Jean Manson for providing mouse brain tissue, and James Ironside for his critical reading of the manuscript at an early stage.
This report is independent research commissioned and funded by the United Kingdom’s Department of Health Policy Research Programme and the Government of Scotland. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health or the Government of Scotland.
Author contributions: The study was conceived and designed by M.A.B. and M.W.H. The experiments were conducted by M.A.B. and A.L. Chronic wasting disease brain specimens were provided by G.M. The manuscript was written by M.A.B. and M.W.H. All authors contributed to the editing and revision of the manuscript.
Prion 2017 Conference Abstracts
First evidence of intracranial and peroral transmission of Chronic Wasting Disease (CWD) into Cynomolgus macaques: a work in progress Stefanie Czub1, Walter Schulz-Schaeffer2, Christiane Stahl-Hennig3, Michael Beekes4, Hermann Schaetzl5 and Dirk Motzkus6 1
University of Calgary Faculty of Veterinary Medicine/Canadian Food Inspection Agency; 2Universitatsklinikum des Saarlandes und Medizinische Fakultat der Universitat des Saarlandes; 3 Deutsches Primaten Zentrum/Goettingen; 4 Robert-Koch-Institut Berlin; 5 University of Calgary Faculty of Veterinary Medicine; 6 presently: Boehringer Ingelheim Veterinary Research Center; previously: Deutsches Primaten Zentrum/Goettingen
This is a progress report of a project which started in 2009.
21 cynomolgus macaques were challenged with characterized CWD material from white-tailed deer (WTD) or elk by intracerebral (ic), oral, and skin exposure routes. Additional blood transfusion experiments are supposed to assess the CWD contamination risk of human blood product. Challenge materials originated from symptomatic cervids for ic, skin scarification and partially per oral routes (WTD brain). Challenge material for feeding of muscle derived from preclinical WTD and from preclinical macaques for blood transfusion experiments. We have confirmed that the CWD challenge material contained at least two different CWD agents (brain material) as well as CWD prions in muscle-associated nerves.
Here we present first data on a group of animals either challenged ic with steel wires or per orally and sacrificed with incubation times ranging from 4.5 to 6.9 years at postmortem. Three animals displayed signs of mild clinical disease, including anxiety, apathy, ataxia and/or tremor. In four animals wasting was observed, two of those had confirmed diabetes. All animals have variable signs of prion neuropathology in spinal cords and brains and by supersensitive IHC, reaction was detected in spinal cord segments of all animals. Protein misfolding cyclic amplification (PMCA), real-time quaking-induced conversion (RT-QuiC) and PET-blot assays to further substantiate these findings are on the way, as well as bioassays in bank voles and transgenic mice.
At present, a total of 10 animals are sacrificed and read-outs are ongoing. Preclinical incubation of the remaining macaques covers a range from 6.4 to 7.10 years. Based on the species barrier and an incubation time of > 5 years for BSE in macaques and about 10 years for scrapie in macaques, we expected an onset of clinical disease beyond 6 years post inoculation.
PRION 2017 DECIPHERING NEURODEGENERATIVE DISORDERS ABSTRACTS REFERENCE
8. Even though human TSE‐exposure risk through consumption of game from European cervids can be assumed to be minor, if at all existing, no final conclusion can be drawn due to the overall lack of scientific data. In particular the US data do not clearly exclude the possibility of human (sporadic or familial) TSE development due to consumption of venison. The Working Group thus recognizes a potential risk to consumers if a TSE would be present in European cervids. It might be prudent considering appropriate measures to reduce such a risk, e.g. excluding tissues such as CNS and lymphoid tissues from the human food chain, which would greatly reduce any potential risk for consumers. However, it is stressed that currently, no data regarding a risk of TSE infections from cervid products are available.
SATURDAY, FEBRUARY 23, 2019
Chronic Wasting Disease CWD TSE Prion and THE FEAST 2003 CDC an updated review of the science 2019
TUESDAY, NOVEMBER 04, 2014
Six-year follow-up of a point-source exposure to CWD contaminated venison in an Upstate New York community: risk behaviours and health outcomes 2005–2011
Authors, though, acknowledged the study was limited in geography and sample size and so it couldn't draw a conclusion about the risk to humans. They recommended more study. Dr. Ermias Belay was the report's principal author but he said New York and Oneida County officials are following the proper course by not launching a study. "There's really nothing to monitor presently. No one's sick," Belay said, noting the disease's incubation period in deer and elk is measured in years. "
Transmission Studies
Mule deer transmissions of CWD were by intracerebral inoculation and compared with natural cases {the following was written but with a single line marked through it ''first passage (by this route)}....TSS
resulted in a more rapidly progressive clinical disease with repeated episodes of synocopy ending in coma. One control animal became affected, it is believed through contamination of inoculum (?saline). Further CWD transmissions were carried out by Dick Marsh into ferret, mink and squirrel monkey. Transmission occurred in ALL of these species with the shortest incubation period in the ferret.
snip....
Prion Infectivity in Fat of Deer with Chronic Wasting Disease▿
Brent Race#, Kimberly Meade-White#, Richard Race and Bruce Chesebro* + Author Affiliations
In mice, prion infectivity was recently detected in fat. Since ruminant fat is consumed by humans and fed to animals, we determined infectivity titers in fat from two CWD-infected deer. Deer fat devoid of muscle contained low levels of CWD infectivity and might be a risk factor for prion infection of other species.
Prions in Skeletal Muscles of Deer with Chronic Wasting Disease
Here bioassays in transgenic mice expressing cervid prion protein revealed the presence of infectious prions in skeletal muscles of CWD-infected deer, demonstrating that humans consuming or handling meat from CWD-infected deer are at risk to prion exposure.
***> now, let’s see what the authors said about this casual link, personal communications years ago, and then the latest on the zoonotic potential from CWD to humans from the TOKYO PRION 2016 CONFERENCE.
see where it is stated NO STRONG evidence. so, does this mean there IS casual evidence ???? “Our conclusion stating that we found no strong evidence of CWD transmission to humans”
From: TSS
Subject: CWD aka MAD DEER/ELK TO HUMANS ???
Date: September 30, 2002 at 7:06 am PST
From: "Belay, Ermias"
To: Cc: "Race, Richard (NIH)" ; ; "Belay, Ermias"
Sent: Monday, September 30, 2002 9:22 AM
Subject: RE: TO CDC AND NIH - PUB MED- 3 MORE DEATHS - CWD - YOUNG HUNTERS
Dear Sir/Madam,
In the Archives of Neurology you quoted (the abstract of which was attached to your email), we did not say CWD in humans will present like variant CJD.. That assumption would be wrong. I encourage you to read the whole article and call me if you have questions or need more clarification (phone: 404-639-3091). Also, we do not claim that "no-one has ever been infected with prion disease from eating venison." Our conclusion stating that we found no strong evidence of CWD transmission to humans in the article you quoted or in any other forum is limited to the patients we investigated.
Ermias Belay, M.D. Centers for Disease Control and Prevention
-----Original Message-----
From: Sent: Sunday, September 29, 2002 10:15 AM
Subject: TO CDC AND NIH - PUB MED- 3 MORE DEATHS - CWD - YOUNG HUNTERS
Sunday, November 10, 2002 6:26 PM .......snip........end..............TSS
Thursday, April 03, 2008
A prion disease of cervids: Chronic wasting disease 2008 1: Vet Res. 2008 Apr 3;39(4):41 A prion disease of cervids: Chronic wasting disease Sigurdson CJ.
snip...
*** twenty-seven CJD patients who regularly consumed venison were reported to the Surveillance Center***,
snip... full text ;
> However, to date, no CWD infections have been reported in people.
sporadic, spontaneous CJD, 85%+ of all human TSE, did not just happen. never in scientific literature has this been proven.
if one looks up the word sporadic or spontaneous at pubmed, you will get a laundry list of disease that are classified in such a way;
sporadic = 54,983 hits https://www.ncbi.nlm.nih.gov/pubmed/?term=sporadic
spontaneous = 325,650 hits https://www.ncbi.nlm.nih.gov/pubmed/?term=spontaneous
key word here is 'reported'. science has shown that CWD in humans will look like sporadic CJD. SO, how can one assume that CWD has not already transmitted to humans? they can't, and it's as simple as that. from all recorded science to date, CWD has already transmitted to humans, and it's being misdiagnosed as sporadic CJD. ...terry
*** LOOKING FOR CWD IN HUMANS AS nvCJD or as an ATYPICAL CJD, LOOKING IN ALL THE WRONG PLACES $$$ ***
> However, to date, no CWD infections have been reported in people.
key word here is ‘reported’. science has shown that CWD in humans will look like sporadic CJD. SO, how can one assume that CWD has not already transmitted to humans? they can’t, and it’s as simple as that. from all recorded science to date, CWD has already transmitted to humans, and it’s being misdiagnosed as sporadic CJD. …terry
*** LOOKING FOR CWD IN HUMANS AS nvCJD or as an ATYPICAL CJD, LOOKING IN ALL THE WRONG PLACES $$$ ***
*** These results would seem to suggest that CWD does indeed have zoonotic potential, at least as judged by the compatibility of CWD prions and their human PrPC target. Furthermore, extrapolation from this simple in vitro assay suggests that if zoonotic CWD occurred, it would most likely effect those of the PRNP codon 129-MM genotype and that the PrPres type would be similar to that found in the most common subtype of sCJD (MM1).***
CWD TSE PRION AND ZOONOTIC, ZOONOSIS, POTENTIAL
Subject: Re: DEER SPONGIFORM ENCEPHALOPATHY SURVEY & HOUND STUDY
Date: Fri, 18 Oct 2002 23:12:22 +0100
From: Steve Dealler
Reply-To: Bovine Spongiform Encephalopathy Organization: Netscape Online member
To: BSE-L@ References: <3daf5023 .4080804="" wt.net="">
Dear Terry,
An excellent piece of review as this literature is desparately difficult to get back from Government sites.
What happened with the deer was that an association between deer meat eating and sporadic CJD was found in about 1993. The evidence was not great but did not disappear after several years of asking CJD cases what they had eaten. I think that the work into deer disease largely stopped because it was not helpful to the UK industry...and no specific cases were reported. Well, if you dont look adequately like they are in USA currenly then you wont find any!
Steve Dealler ===============
''The association between venison eating and risk of CJD shows similar pattern, with regular venison eating associated with a 9 FOLD INCREASE IN RISK OF CJD (p = 0.04).''
CREUTZFELDT JAKOB DISEASE SURVEILLANCE IN THE UNITED KINGDOM THIRD ANNUAL REPORT AUGUST 1994
Consumption of venison and veal was much less widespread among both cases and controls. For both of these meats there was evidence of a trend with increasing frequency of consumption being associated with increasing risk of CJD. (not nvCJD, but sporadic CJD...tss) These associations were largely unchanged when attention was restricted to pairs with data obtained from relatives. ...
Table 9 presents the results of an analysis of these data.
There is STRONG evidence of an association between ‘’regular’’ veal eating and risk of CJD (p = .0.01).
Individuals reported to eat veal on average at least once a year appear to be at 13 TIMES THE RISK of individuals who have never eaten veal.
There is, however, a very wide confidence interval around this estimate. There is no strong evidence that eating veal less than once per year is associated with increased risk of CJD (p = 0.51).
The association between venison eating and risk of CJD shows similar pattern, with regular venison eating associated with a 9 FOLD INCREASE IN RISK OF CJD (p = 0.04).
There is some evidence that risk of CJD INCREASES WITH INCREASING FREQUENCY OF LAMB EATING (p = 0.02).
The evidence for such an association between beef eating and CJD is weaker (p = 0.14). When only controls for whom a relative was interviewed are included, this evidence becomes a little STRONGER (p = 0.08).
snip...
It was found that when veal was included in the model with another exposure, the association between veal and CJD remained statistically significant (p = < 0.05 for all exposures), while the other exposures ceased to be statistically significant (p = > 0.05).
snip...
In conclusion, an analysis of dietary histories revealed statistical associations between various meats/animal products and INCREASED RISK OF CJD. When some account was taken of possible confounding, the association between VEAL EATING AND RISK OF CJD EMERGED AS THE STRONGEST OF THESE ASSOCIATIONS STATISTICALLY. ...
snip...
In the study in the USA, a range of foodstuffs were associated with an increased risk of CJD, including liver consumption which was associated with an apparent SIX-FOLD INCREASE IN THE RISK OF CJD. By comparing the data from 3 studies in relation to this particular dietary factor, the risk of liver consumption became non-significant with an odds ratio of 1.2 (PERSONAL COMMUNICATION, PROFESSOR A. HOFMAN. ERASMUS UNIVERSITY, ROTTERDAM). (???...TSS)
snip...see full report ;
http://web.archive.org/web/20090506050043/http://www.bseinquiry.gov.uk/files/yb/1994/08/00004001.pdf
http://web.archive.org/web/20090506050244/http://www.bseinquiry.gov.uk/files/yb/1994/07/00001001.pdf
BSE Inquiry Steve Dealler
Management In Confidence
BSE: Private Submission of Bovine Brain Dealler
snip...see full text;
MONDAY, FEBRUARY 25, 2019
***> MAD DOGS AND ENGLISHMEN BSE, SCRAPIE, CWD, CJD, TSE PRION A REVIEW 2019
***> ''The association between venison eating and risk of CJD shows similar pattern, with regular venison eating associated with a 9 FOLD INCREASE IN RISK OF CJD (p = 0.04).''
***> In conclusion, sensory symptoms and loss of reflexes in Gerstmann-Sträussler-Scheinker syndrome can be explained by neuropathological changes in the spinal cord. We conclude that the sensory symptoms and loss of lower limb reflexes in Gerstmann-Sträussler-Scheinker syndrome is due to pathology in the caudal spinal cord. <***
***> The clinical and pathological presentation in macaques was mostly atypical, with a strong emphasis on spinal cord pathology.<***
***> The notion that CWD can be transmitted orally into both new-world and old-world non-human primates asks for a careful reevaluation of the zoonotic risk of CWD. <***
***> All animals have variable signs of prion neuropathology in spinal cords and brains and by supersensitive IHC, reaction was detected in spinal cord segments of all animals.<***
***> In particular the US data do not clearly exclude the possibility of human (sporadic or familial) TSE development due to consumption of venison. The Working Group thus recognizes a potential risk to consumers if a TSE would be present in European cervids.'' Scientific opinion on chronic wasting disease (II) <***
***Moreover, sporadic disease has never been observed in breeding colonies or primate research laboratories, most notably among hundreds of animals over several decades of study at the National Institutes of Health25, and in nearly twenty older animals continuously housed in our own facility.***
Even if the prevailing view is that sporadic CJD is due to the spontaneous formation of CJD prions, it remains possible that its apparent sporadic nature may, at least in part, result from our limited capacity to identify an environmental origin.
https://www.nature.com/articles/srep11573
O.05: Transmission of prions to primates after extended silent incubation periods: Implications for BSE and scrapie risk assessment in human populations
Emmanuel Comoy, Jacqueline Mikol, Valerie Durand, Sophie Luccantoni, Evelyne Correia, Nathalie Lescoutra, Capucine Dehen, and Jean-Philippe Deslys Atomic Energy Commission; Fontenay-aux-Roses, France
Prion diseases (PD) are the unique neurodegenerative proteinopathies reputed to be transmissible under field conditions since decades. The transmission of Bovine Spongiform Encephalopathy (BSE) to humans evidenced that an animal PD might be zoonotic under appropriate conditions. Contrarily, in the absence of obvious (epidemiological or experimental) elements supporting a transmission or genetic predispositions, PD, like the other proteinopathies, are reputed to occur spontaneously (atpical animal prion strains, sporadic CJD summing 80% of human prion cases).
Non-human primate models provided the first evidences supporting the transmissibiity of human prion strains and the zoonotic potential of BSE. Among them, cynomolgus macaques brought major information for BSE risk assessment for human health (Chen, 2014), according to their phylogenetic proximity to humans and extended lifetime. We used this model to assess the zoonotic potential of other animal PD from bovine, ovine and cervid origins even after very long silent incubation periods.
*** We recently observed the direct transmission of a natural classical scrapie isolate to macaque after a 10-year silent incubation period,
***with features similar to some reported for human cases of sporadic CJD, albeit requiring fourfold long incubation than BSE. Scrapie, as recently evoked in humanized mice (Cassard, 2014),
***is the third potentially zoonotic PD (with BSE and L-type BSE),
***thus questioning the origin of human sporadic cases.
We will present an updated panorama of our different transmission studies and discuss the implications of such extended incubation periods on risk assessment of animal PD for human health.
===============
***thus questioning the origin of human sporadic cases***
===============
***our findings suggest that possible transmission risk of H-type BSE to sheep and human. Bioassay will be required to determine whether the PMCA products are infectious to these animals.
==============
https://prion2015.files.wordpress.com/2015/05/prion2015abstracts.pdf
***Transmission data also revealed that several scrapie prions propagate in HuPrP-Tg mice with efficiency comparable to that of cattle BSE. While the efficiency of transmission at primary passage was low, subsequent passages resulted in a highly virulent prion disease in both Met129 and Val129 mice.
***Transmission of the different scrapie isolates in these mice leads to the emergence of prion strain phenotypes that showed similar characteristics to those displayed by MM1 or VV2 sCJD prion.
***These results demonstrate that scrapie prions have a zoonotic potential and raise new questions about the possible link between animal and human prions.
http://www.tandfonline.com/doi/abs/10.1080/19336896.2016.1163048?journalCode=kprn20
Prion diseases (PD) are the unique neurodegenerative proteinopathies reputed to be transmissible under field conditions since decades. The transmission of Bovine Spongiform Encephalopathy (BSE) to humans evidenced that an animal PD might be zoonotic under appropriate conditions. Contrarily, in the absence of obvious (epidemiological or experimental) elements supporting a transmission or genetic predispositions, PD, like the other proteinopathies, are reputed to occur spontaneously (atpical animal prion strains, sporadic CJD summing 80% of human prion cases).
Non-human primate models provided the first evidences supporting the transmissibiity of human prion strains and the zoonotic potential of BSE. Among them, cynomolgus macaques brought major information for BSE risk assessment for human health (Chen, 2014), according to their phylogenetic proximity to humans and extended lifetime. We used this model to assess the zoonotic potential of other animal PD from bovine, ovine and cervid origins even after very long silent incubation periods.
*** We recently observed the direct transmission of a natural classical scrapie isolate to macaque after a 10-year silent incubation period,
***with features similar to some reported for human cases of sporadic CJD, albeit requiring fourfold long incubation than BSE. Scrapie, as recently evoked in humanized mice (Cassard, 2014),
***is the third potentially zoonotic PD (with BSE and L-type BSE),
***thus questioning the origin of human sporadic cases.
We will present an updated panorama of our different transmission studies and discuss the implications of such extended incubation periods on risk assessment of animal PD for human health.
===============
***thus questioning the origin of human sporadic cases***
===============
***our findings suggest that possible transmission risk of H-type BSE to sheep and human. Bioassay will be required to determine whether the PMCA products are infectious to these animals.
==============
https://prion2015.files.wordpress.com/2015/05/prion2015abstracts.pdf
***Transmission data also revealed that several scrapie prions propagate in HuPrP-Tg mice with efficiency comparable to that of cattle BSE. While the efficiency of transmission at primary passage was low, subsequent passages resulted in a highly virulent prion disease in both Met129 and Val129 mice.
***Transmission of the different scrapie isolates in these mice leads to the emergence of prion strain phenotypes that showed similar characteristics to those displayed by MM1 or VV2 sCJD prion.
***These results demonstrate that scrapie prions have a zoonotic potential and raise new questions about the possible link between animal and human prions.
http://www.tandfonline.com/doi/abs/10.1080/19336896.2016.1163048?journalCode=kprn20
PRION 2016 TOKYO
Saturday, April 23, 2016
SCRAPIE WS-01: Prion diseases in animals and zoonotic potential 2016
Prion. 10:S15-S21. 2016 ISSN: 1933-6896 printl 1933-690X online
Taylor & Francis
Prion 2016 Animal Prion Disease Workshop Abstracts
WS-01: Prion diseases in animals and zoonotic potential
Transmission of the different scrapie isolates in these mice leads to the emergence of prion strain phenotypes that showed similar characteristics to those displayed by MM1 or VV2 sCJD prion.
These results demonstrate that scrapie prions have a zoonotic potential and raise new questions about the possible link between animal and human prions.
http://www.tandfonline.com/doi/abs/10.1080/19336896.2016.1163048?journalCode=kprn20
Title: Transmission of scrapie prions to primate after an extended silent incubation period)
*** In complement to the recent demonstration that humanized mice are susceptible to scrapie, we report here the first observation of direct transmission of a natural classical scrapie isolate to a macaque after a 10-year incubation period. Neuropathologic examination revealed all of the features of a prion disease: spongiform change, neuronal loss, and accumulation of PrPres throughout the CNS.
*** This observation strengthens the questioning of the harmlessness of scrapie to humans, at a time when protective measures for human and animal health are being dismantled and reduced as c-BSE is considered controlled and being eradicated.
*** Our results underscore the importance of precautionary and protective measures and the necessity for long-term experimental transmission studies to assess the zoonotic potential of other animal prion strains.
http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=313160
1: J Infect Dis 1980 Aug;142(2):205-8
Oral transmission of kuru, Creutzfeldt-Jakob disease, and scrapie to nonhuman primates.
Gibbs CJ Jr, Amyx HL, Bacote A, Masters CL, Gajdusek DC.
Kuru and Creutzfeldt-Jakob disease of humans and scrapie disease of sheep and goats were transmitted to squirrel monkeys (Saimiri sciureus) that were exposed to the infectious agents only by their nonforced consumption of known infectious tissues. The asymptomatic incubation period in the one monkey exposed to the virus of kuru was 36 months; that in the two monkeys exposed to the virus of Creutzfeldt-Jakob disease was 23 and 27 months, respectively; and that in the two monkeys exposed to the virus of scrapie was 25 and 32 months, respectively. Careful physical examination of the buccal cavities of all of the monkeys failed to reveal signs or oral lesions. One additional monkey similarly exposed to kuru has remained asymptomatic during the 39 months that it has been under observation.
snip...
The successful transmission of kuru, Creutzfeldt-Jakob disease, and scrapie by natural feeding to squirrel monkeys that we have reported provides further grounds for concern that scrapie-infected meat may occasionally give rise in humans to Creutzfeldt-Jakob disease.
PMID: 6997404
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6997404&dopt=Abstract
Recently the question has again been brought up as to whether scrapie is transmissible to man. This has followed reports that the disease has been transmitted to primates. One particularly lurid speculation (Gajdusek 1977) conjectures that the agents of scrapie, kuru, Creutzfeldt-Jakob disease and transmissible encephalopathy of mink are varieties of a single "virus". The U.S. Department of Agriculture concluded that it could "no longer justify or permit scrapie-blood line and scrapie-exposed sheep and goats to be processed for human or animal food at slaughter or rendering plants" (ARC 84/77)" The problem is emphasised by the finding that some strains of scrapie produce lesions identical to the once which characterise the human dementias"
Whether true or not. the hypothesis that these agents might be transmissible to man raises two considerations. First, the safety of laboratory personnel requires prompt attention. Second, action such as the "scorched meat" policy of USDA makes the solution of the scrapie problem urgent if the sheep industry is not to suffer grievously.
snip...
76/10.12/4.6
Nature. 1972 Mar 10;236(5341):73-4.
Transmission of scrapie to the cynomolgus monkey (Macaca fascicularis).
Gibbs CJ Jr, Gajdusek DC.
Nature 236, 73 - 74 (10 March 1972); doi:10.1038/236073a0
Transmission of Scrapie to the Cynomolgus Monkey (Macaca fascicularis)
C. J. GIBBS jun. & D. C. GAJDUSEK
National Institute of Neurological Diseases and Stroke, National Institutes of Health, Bethesda, Maryland
SCRAPIE has been transmitted to the cynomolgus, or crab-eating, monkey (Macaca fascicularis) with an incubation period of more than 5 yr from the time of intracerebral inoculation of scrapie-infected mouse brain. The animal developed a chronic central nervous system degeneration, with ataxia, tremor and myoclonus with associated severe scrapie-like pathology of intensive astroglial hypertrophy and proliferation, neuronal vacuolation and status spongiosus of grey matter. The strain of scrapie virus used was the eighth passage in Swiss mice (NIH) of a Compton strain of scrapie obtained as ninth intracerebral passage of the agent in goat brain, from Dr R. L. Chandler (ARC, Compton, Berkshire).
Wednesday, February 16, 2011
IN CONFIDENCE
SCRAPIE TRANSMISSION TO CHIMPANZEES
IN CONFIDENCE
reference...
RB3.20
TRANSMISSION TO CHIMPANZEES
1. Kuru and CJD have been successfully transmitted to chimpanzees but scrapie and TME have not.
2. We cannot say that scrapie will not transmit to chimpanzees. There are several scrapie strains and I am not aware that all have been tried (that would have to be from mouse passaged material). Nor has a wide enough range of field isolates subsequently strain typed in mice been inoculated by the appropriate routes (i/c, ilp and i/v) :
3. I believe the proposed experiment to determine transmissibility, if conducted, would only show the susceptibility or resistance of the chimpanzee to infection/disease by the routes used and the result could not be interpreted for the predictability of the susceptibility for man. Proposals for prolonged oral exposure of chimpanzees to milk from cattle were suggested a long while ago and rejected.
4. In view of Dr Gibbs' probable use of chimpazees Mr Wells' comments (enclosed) are pertinent. I have yet to receive a direct communication from Dr Schellekers but before any collaboration or provision of material we should identify the Gibbs' proposals and objectives.
5. A positive result from a chimpanzee challenged severely would likely create alarm in some circles even if the result could not be interpreted for man. I have a view that all these agents could be transmitted provided a large enough dose by appropriate routes was given and the animals kept long enough. Until the mechanisms of the species barrier are more clearly understood it might be best to retain that hypothesis.
6. A negative result would take a lifetime to determine but that would be a shorter period than might be available for human exposure and it would still not answer the question regarding mans' susceptibility. In the meantime no doubt the negativity would be used defensively. It would however be counterproductive if the experiment finally became positive. We may learn more about public reactions following next Monday' s meeting.
R. Bradley
23 September 1990
CVO (+Mr Wells' comments)
Dr T W A Little
Dr B J Shreeve
90/9.23/1.1.
http://web.archive.org/web/20090506041740/http://www.bseinquiry.gov.uk/files/yb/1990/09/23001001.pdf
IN CONFIDENCE CHIMPANZEES
CODE 18-77 Reference RB3.46
Some further information that may assist in decision making has been gained by discussion with Dr Rosalind Ridley.
She says that careful study of Gajdusek's work shows no increased susceptibility of chimpanzees over New World Monkeys such as Squirrel Monkeys. She does not think it would tell you anything about the susceptibility to man. Also Gajdusek did not, she believes, challenge chimpanzees with scrapie as severely as we did pigs and we know little of that source of scrapie. Comparisons would be difficult. She also would not expect the Home Office to sanction such experiments here unless there was a very clear and important objective that would be important for human health protection. She doubted such a case could be made. If this is the case she thought it would be unethical to do an experiment abroad because we could not do it in our own country.
Retrospectively she feels they should have put up more marmosets than they did. They all remain healthy. They would normally regard the transmission as negative if no disease resulted in five years.
We are not being asked for a decision but I think that before we made one we should gain as much knowledge as we can. If we decided to proceed we would have to bear any criticisms for many years if there was an adverse view by scientists ormedia. This should not be undertaken lightly. There is already some adverse comment here, I gather, on the pig experiment though that will subside.
The Gibbs' (as' distinct from Schellekers') study is somewhat different. We are merely supplying material for comparative studies in a laboratory with the greatest experience of human SEs in the world and it has been sanctioned by USDA (though we do not know for certain yet if chimpanzees specifically will be used). This would keep it at a lower profile than if we conducted such an experiment in the UK or Europe.
I consider we must have very powerful and defendable objectives to go beyond Gibbs' proposed experiments and should not initiate others just because an offer has been made.
Scientists have a responsibility to seek other methods of investigative research other than animal experimentation. At present no objective has convinced me we need to do research using Chimpanzees - a species in need of protection. Resisting such proposals would enable us to communicate that information to the scientist and the public should the need arise. A line would have been drawn.
CVO cc Dr T Dr B W A Little Dr B J Shreeve
R Bradley
26 September 1990
90/9.26/3.2
http://web.archive.org/web/20090506041605/http://www.bseinquiry.gov.uk/files/yb/1990/09/26003001.pdf
this is tse prion political theater here, i.e. what i call TSE PRION POKER...tss
3. Prof. A. Robertson gave a brief account of BSE. The US approach was to accord it a very low profile indeed. Dr. A Thiermann showed the picture in the ''Independent'' with cattle being incinerated and thought this was a fanatical incident to be avoided in the US at all costs.
snip...
PAGE 26
Transmission Studies
Mule deer transmissions of CWD were by intracerebral inoculation and compared with natural cases {the following was written but with a single line marked through it ''first passage (by this route)}....TSS
resulted in a more rapidly progressive clinical disease with repeated episodes of synocopy ending in coma. One control animal became affected, it is believed through contamination of inoculum (?saline). Further CWD transmissions were carried out by Dick Marsh into ferret, mink and squirrel monkey. Transmission occurred in ALL of these species with the shortest incubation period in the ferret.
The occurrence of CWD must be viewed against the contest of the locations in which it occurred. It was an incidental and unwelcome complication of the respective wildlife research programmes. Despite its subsequent recognition as a new disease of cervids, therefore justifying direct investigation, no specific research funding was forthcoming. The USDA veiwed it as a wildlife problem and consequently not their province! ...page 26.
snip...see;
IN CONFIDENCE
PERCEPTIONS OF UNCONVENTIONAL SLOW VIRUS DISEASE OF ANIMALS IN THE USA
GAH WELLS
REPORT OF A VISIT TO THE USA
APRIL-MAY 1989
TUESDAY, MAY 11, 2021
***> A Unique Presentation of Creutzfeldt-Jakob Disease in a Patient Consuming Deer Antler Velvet <***
Saturday, May 1, 2021
***> Clinical Use of Improved Diagnostic Testing for Detection of Prion Disease <***
FRIDAY, MAY 14, 2021
Texas CWD TSE Prion Discovered at Deer Breeding Facilities in Matagorda and Mason Counties With 228 Positive To Date Total
“Regrettably, the gravity of this situation continues to mount with these new CWD positive discoveries, as well as with the full understanding of just how many other facilities and release sites across Texas were connected to the CWD positive sites in Uvalde and Hunt Counties,” said Carter Smith, Executive Director of TPWD.
WEDNESDAY, MARCH 24, 2021
USDA Animal and Plant Health Inspection Service 2020 IMPACT REPORT BSE TSE Prion Testing and Surveillance MIA
https://animalhealthreportpriontse.blogspot.com/2021/03/usda-animal-and-plant-health-inspection.html
WEDNESDAY, DECEMBER 2, 2020
EFSA Evaluation of public and animal health risks in case of a delayed post-mortem inspection in ungulates EFSA Panel on Biological Hazards (BIOHAZ) ADOPTED: 21 October 2020
i wonder if a 7 month delay on a suspect BSE case in Texas is too long, on a 48 hour turnaround, asking for a friend???
MONDAY, NOVEMBER 30, 2020
***> REPORT OF THE MEETING OF THE OIE SCIENTIFIC COMMISSION FOR ANIMAL DISEASES Paris, 9–13 September 2019 BSE, TSE, PRION
see updated concerns with atypical BSE from feed and zoonosis...terry
WEDNESDAY, DECEMBER 23, 2020
BSE research project final report 2005 to 2008 SE1796 SID5
Terry S. Singeltary Sr.